
In Figure P19.22, the change in internal energy of a gas that is taken from A to C along the blue path is +800 J. The work done on the gas along the red path ABC is −500 J. (a) How much energy must be added to the system by heat as it goes from A through B to C? (b) If the pressure at point A is five times that of point C, what is the work done on the system in going from C to D?
Figure P19.22
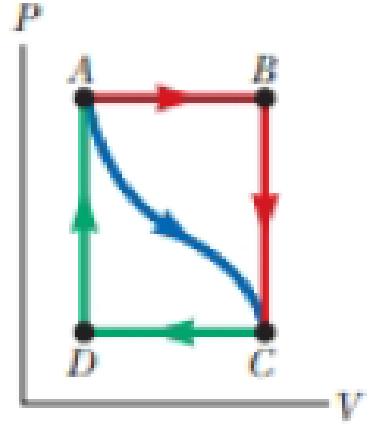
(c) What is the energy exchanged with the surroundings by heat as the gas goes from C to A along the green path? (d) If the change in internal energy in going from point D to point A is +500 J, how much energy must be added to the system by heat as it goes from point C to point D?
(a)
The energy which must be added to the system by heat which goes from A through B to C.
Answer to Problem 22P
The energy which must be added to the system by heat which goes from A through B to C is
Explanation of Solution
Given info: The change in internal energy of a gas which is taken along the blue path from A to C is
Write the equation of first law of thermodynamics.
Here,
Write the equation of conservation of energy.
Here,
Substitute
Substitute
Conclusion:
Therefore, the energy which must be added to the system by heat which goes from A through B to C is
(b)
The work done on the system from C to D if pressure at A is five times that of point C.
Answer to Problem 22P
The work done on the system from C to D if pressure at A is five times that of point C is
Explanation of Solution
Given info: The change in internal energy of a gas which is taken along the blue path from A to C is
Write the equation to calculate the work done on the system from C to D.
Here,
Here,
The pressure at point A is five times that of point C.
And the volume is,
Substitute
Substitute
Conclusion:
Therefore, the work done on the system from C to D if pressure at A is five times that of point C is
(c)
The energy exchanged with the surroundings by heat along the green path from C to A.
Answer to Problem 22P
The energy exchanged with the surroundings by heat along the green path from C to A is
Explanation of Solution
Given info: The change in internal energy of a gas which is taken along the blue path from A to C is
Write the equation of first law of thermodynamics.
Here,
Write the equation to calculate work done along the green path from C to A.
Here, the volume
Substitute 0 for
Substitute
Substitute
Conclusion:
Therefore, the energy exchanged with the surroundings by heat along the green path from C to A is
(d)
The energy which must be added to the system by heat which goes from C to D.
Answer to Problem 22P
The energy which must be added to the system by heat which goes from C to D
is
Explanation of Solution
Given info: The change in internal energy of a gas which is taken along the blue path from A to C is
Write the equation of first law of thermodynamics.
Here,
Substitute
Conclusion:
Therefore, the energy which must be added to the system by heat which goes from C to D is
Want to see more full solutions like this?
Chapter 19 Solutions
Bundle: Physics For Scientists And Engineers With Modern Physics, Loose-leaf Version, 10th + Webassign Printed Access Card For Serway/jewett's Physics For Scientists And Engineers, 10th, Single-term
- In Figure P17.32, the change in internal energy of a gas that is taken from A to C along the blue path is +800 J. The work done on the gas along the red path ABC is 500 J. (a) How much energy must be added to the system by heat as it goes from A through B to C? (b) If the pressure at point A is five times that of point C, what is the work done on the system in going from C to D? (c) What is the energy exchanged with the surroundings by heat as the gas goes from C to A along the green path? (d) If the change in internal energy in going from point D to point A is +500 J, how much energy must be added to the system by heat as it goes from point C to point D? Figure P17.32arrow_forwardIf a gas is compressed isothermally, which of the following statements is true? (a) Energy is transferred into the gas by heat. (b) No work is done on the gas. (c) The temperature of the gas increases. (d) The internal energy of the gas remains constant. (e) None of those statements is true.arrow_forwardIn Figure P19.22, the change in internal energy of a gas that is taken from A to C along the blue path is +800 J. The work done on the gas along the red path ABC is 500 J. (a) How much energy must be added to the system by heat as it goes from A through B to C? (b) If the pressure at point A is five times that of point C, what is the work done on the system in going from C to D? Figure P19.22 (c) What is the energy exchanged with the surroundings by heat as the gas goes from C to A along the green path? (d) If the change in internal energy in going from point D to point A is +500 J, how much energy must be added to the system by heat as it goes from point C to point D?arrow_forward
- One mole of an ideal gas does 3 000 J of work on its surroundings as it expands isothermally to a final pressure of 1.00 atm and volume of 25.0 L. Determine (a) the initial volume and (b) the temperature of the gas.arrow_forwardA 2.00-mol sample of a diatomic ideal gas expands slowly and adiabatically from a pressure of 5.00 atm and a volume of 12.0 L to a final volume of 30.0 L. (a) What is the final pressure of the gas? (b) What are the initial and final temperatures? Find (c) Q, (d) Eint, and (e) W for the gas during this process.arrow_forwardA gas expands from I to Fin Figure P20.58 (page 622). The energy added to the gas by heat is 418 J when the gas goes from I to F along the diagonal path, (a) What is the change in internal energy of the gas? (b) How much energy must be added to the gas by heat along the indirect path IAF?arrow_forward
- Consider a gas filling two connected chambers that are separated by a removable barrier (Fig. P20.68). The gas molecules on the left (red) are initially at a higher temperature than the ones on the right (blue). When the barrier between the two chambers is removed, the molecules begin to mix and move from one chamber to the other. a. Describe what happens to the temperature in the left chamber and in the right chamber as time goes on, once the barrier is open. Discuss in terms of the mixing of the molecules from each gas. b. Describe what happens to the most probable speed and average speed in the left chamber and in the right chamber as time goes on, once the barrier is open. Do they increase or decrease by the same factor? Explain. FIGURE P20.68 Problems 68 and 69.arrow_forwardA gas in a cylindrical closed container is adiabatically and quasi-statically expanded from a state A (3 MPa, 2 L) to a state B with volume of 6 L along the path 1.8pV= constant. (a) Plot the path in the pV plane. (b) Find the amount of work done by the gas and the change in the internal energy of the gas during the process.arrow_forward(a) Determine the work done on a gas that expands from i to f as indicated in Figure P19.16. (b) What If? How much work is done on the gas if it is compressed from f to i along the same path? Figure P19.16arrow_forward
- An ideal gas with specific heat ratio confined to a cylinder is put through a closed cycle. Initially, the gas is at Pi, Vi, and Ti. First, its pressure is tripled under constant volume. It then expands adiabatically to its original pressure and finally is compressed isobarically to its original volume. (a) Draw a PV diagram of this cycle. (b) Determine the volume at the end of the adiabatic expansion. Find (c) the temperature of the gas at the start of the adiabatic expansion and (d) the temperature at the end of the cycle. (e) What was the net work done on the gas for this cycle?arrow_forwardA thermodynamic cycle is shown in Figure P21.34 for a gas in a piston. The system changes states along the path ABCA. a. What is the total work done by the gas during this cycle? b. How much heat is transferred? Does heat flow into or out of the system? Figure P21.34arrow_forwardOne mole of an ideal monatomic gas occupies a volume of 1.0102 m3 at a pressure of 2.0105 N/m2. (a) What is the temperature of the gas? (b) The gas undergoes a quasi-static adiabatic compression until its volume is decreased to 5.0103 m3. is the new gas temperature? (c) How much work is done on the gas during the compression? (d) What is the change in the internal energy of the gas?arrow_forward
 Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning





