
Bundle: Physics For Scientists And Engineers With Modern Physics, Loose-leaf Version, 10th + Webassign Printed Access Card For Serway/jewett's Physics For Scientists And Engineers, 10th, Single-term
10th Edition
ISBN: 9781337888585
Author: Raymond A. Serway, John W. Jewett
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 19, Problem 16P
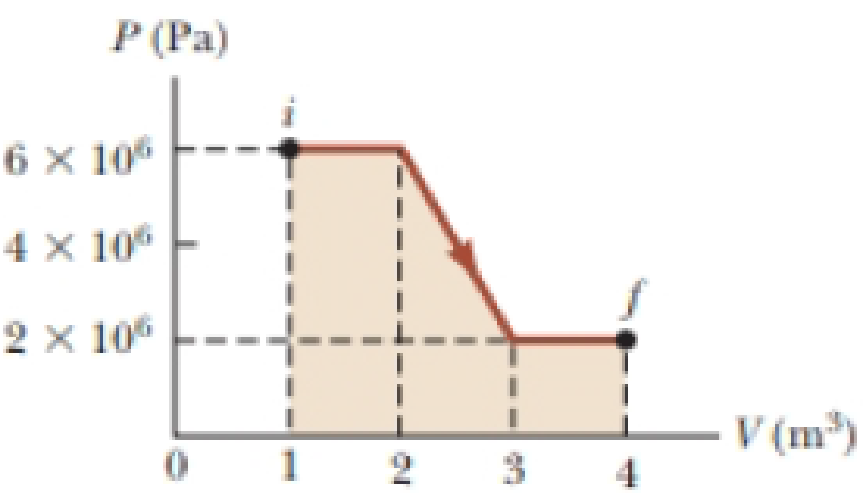
(a) Determine the work done on a gas that expands from i to f as indicated in Figure P19.16. (b) What If? How much work is done on the gas if it is compressed from f to i along the same path?
Figure P19.16
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
In an electron gun, electrons are accelerated through a region with an electric field of magnitude 1.5 × 104 N/C for a distance of 2.5 cm. If the electrons start from rest, how fast are they moving after traversing the gun?
Please solve and answer this problem correctly please. Thank you!!
Please solve and answer this problem correctly please. Thank you!!
Chapter 19 Solutions
Bundle: Physics For Scientists And Engineers With Modern Physics, Loose-leaf Version, 10th + Webassign Printed Access Card For Serway/jewett's Physics For Scientists And Engineers, 10th, Single-term
Ch. 19.2 - Prob. 19.1QQCh. 19.3 - Prob. 19.2QQCh. 19.5 - Prob. 19.3QQCh. 19.5 - Characterize the paths in Figure 19.12 as...Ch. 19.6 - Prob. 19.5QQCh. 19 - Prob. 1PCh. 19 - The highest waterfall in the world is the Salto...Ch. 19 - Prob. 3PCh. 19 - The temperature of a silver bar rises by 10.0C...Ch. 19 - You are working in your kitchen preparing lunch...
Ch. 19 - If water with a mass mk at temperature Tk is...Ch. 19 - Prob. 7PCh. 19 - An electric drill with a steel drill bit of mass m...Ch. 19 - Prob. 9PCh. 19 - How much energy is required to change a 40.0-g ice...Ch. 19 - Prob. 11PCh. 19 - Prob. 12PCh. 19 - In an insulated vessel, 250 g of ice at 0C is...Ch. 19 - Prob. 14PCh. 19 - One mole of an ideal gas is warmed slowly so that...Ch. 19 - (a) Determine the work done on a gas that expands...Ch. 19 - A thermodynamic system undergoes a process in...Ch. 19 - Prob. 18PCh. 19 - A 2.00-mol sample of helium gas initially at 300...Ch. 19 - (a) How much work is done on the steam when 1.00...Ch. 19 - A 1.00-kg block of aluminum is warmed at...Ch. 19 - In Figure P19.22, the change in internal energy of...Ch. 19 - Prob. 23PCh. 19 - A concrete slab is 12.0 cm thick and has an area...Ch. 19 - Two lightbulbs have cylindrical filaments much...Ch. 19 - Prob. 26PCh. 19 - (a) Calculate the R-value of a thermal window made...Ch. 19 - Prob. 28PCh. 19 - Gas in a container is at a pressure of 1.50 atm...Ch. 19 - Prob. 30APCh. 19 - You have a particular interest in automobile...Ch. 19 - Prob. 32APCh. 19 - Prob. 33APCh. 19 - Prob. 34APCh. 19 - Review. Following a collision between a large...Ch. 19 - Prob. 36APCh. 19 - An ice-cube tray is filled with 75.0 g of water....Ch. 19 - Prob. 38APCh. 19 - An iron plate is held against an iron wheel so...Ch. 19 - One mole of an ideal gas is contained in a...Ch. 19 - Prob. 41APCh. 19 - Prob. 42APCh. 19 - Prob. 43APCh. 19 - A student measures the following data in a...Ch. 19 - (a) The inside of a hollow cylinder is maintained...Ch. 19 - Prob. 46CPCh. 19 - Prob. 47CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- a) Use the node-voltage method to find v1, v2, and v3 in the circuit in Fig. P4.14. b) How much power does the 40 V voltage source deliver to the circuit? Figure P4.14 302 202 w w + + + 40 V V1 80 Ω 02 ΣΑΩ 28 A V3 + w w 102 202arrow_forwardPlease solve and answer this problem correctly please. Thank you!!arrow_forwardYou're on an interplanetary mission, in an orbit around the Sun. Suppose you make a maneuver that brings your perihelion in closer to the Sun but leaves your aphelion unchanged. Then you must have Question 2 options: sped up at perihelion sped up at aphelion slowed down at perihelion slowed down at aphelionarrow_forward
- The force of the quadriceps (Fq) and force of the patellar tendon (Fp) is identical (i.e., 1000 N each). In the figure below angle in blue is Θ and the in green is half Θ (i.e., Θ/2). A) Calculate the patellar reaction force (i.e., R resultant vector is the sum of the horizontal component of the quadriceps and patellar tendon force) at the following joint angles: you need to provide a diagram showing the vector and its components for each part. a1) Θ = 160 degrees, a2) Θ = 90 degrees. NOTE: USE ONLY TRIGNOMETRIC FUNCTIONS (SIN/TAN/COS, NO LAW OF COSINES, NO COMPLICATED ALGEBRAIC EQUATIONS OR ANYTHING ELSE, ETC. Question A has 2 parts!arrow_forwardThe force of the quadriceps (Fq) and force of the patellar tendon (Fp) is identical (i.e., 1000 N each). In the figure below angle in blue is Θ and the in green is half Θ (i.e., Θ/2). A) Calculate the patellar reaction force (i.e., R resultant vector is the sum of the horizontal component of the quadriceps and patellar tendon force) at the following joint angles: you need to provide a diagram showing the vector and its components for each part. a1) Θ = 160 degrees, a2) Θ = 90 degrees. NOTE: USE DO NOT USE LAW OF COSINES, NO COMPLICATED ALGEBRAIC EQUATIONS OR ANYTHING ELSE, ETC. Question A has 2 parts!arrow_forwardNo chatgpt pls will upvotearrow_forward
- The force of the quadriceps (Fq) and force of the patellar tendon (Fp) is identical (i.e., 1000 N each). In the figure below angle in blue is Θ and the in green is half Θ (i.e., Θ/2). A) Calculate the patellar reaction force (i.e., R resultant vector is the sum of the horizontal component of the quadriceps and patellar tendon force) at the following joint angles: you need to provide a diagram showing the vector and its components for each part. a1) Θ = 160 degrees, a2) Θ = 90 degrees. NOTE: USE ONLY TRIGNOMETRIC FUNCTIONS (SIN/TAN/COS, NO LAW OF COSINES, NO COMPLICATED ALGEBRAIC EQUATIONS OR ANYTHING ELSE, ETC. Question A has 2 parts!arrow_forwardNo chatgpt pls will upvotearrow_forwardNo chatgpt pls will upvotearrow_forward
- Solve and answer the question correctly please. Thank you!!arrow_forward་ The position of a particle is described by r = (300e 0.5t) mm and 0 = (0.3t²) rad, where t is in seconds. Part A Determine the magnitude of the particle's velocity at the instant t = 1.5 s. Express your answer to three significant figures and include the appropriate units. v = Value Submit Request Answer Part B ? Units Determine the magnitude of the particle's acceleration at the instant t = 1.5 s. Express your answer to three significant figures and include the appropriate units. a = Value A ? Unitsarrow_forwardSolve and answer the question correctly please. Thank you!!arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Thermodynamics: Crash Course Physics #23; Author: Crash Course;https://www.youtube.com/watch?v=4i1MUWJoI0U;License: Standard YouTube License, CC-BY