
Concept explainers
Draw structural formulas for the following amino acids, identify the chiral carbon atom in each one, and circle the four different groups attached to the chiral carbon.
a. valine
b. glutamate
c. asparagine
d. cysteine
(a)
Interpretation:
The structural formula for the amino acid, valine is to be drawn. The chiral carbon atom in it is to be identified. The four different groups attached to the chiral carbon atom are to be circled.
Concept introduction:
Amino acids are organic compounds which combine sequentially to generate a protein. They are known as the building blocks of the human body. The main elements present in amino acids are carbon, nitrogen and oxygen while the side chains attached to the chiral carbon atom contain other elements.
Answer to Problem 19.5E
The structural formula for the amino acid, valine is shown below.
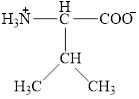
The chiral carbon atom in it is shown below.
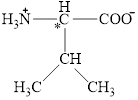
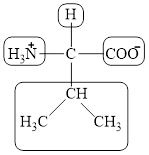
The four different groups attached to the chiral carbon atom are circled as shown below.
Explanation of Solution
Amino acids are formed by an amine and a carboxylic acid attached to a carbon atom with a characteristic side chain. The carboxylic acid in amino acids is usually in an ionic form and therefore, it is known as carboxylate group.
The structure of valine is given below.

Figure 1
The chiral carbon atom in it is shown below.

Figure 2
The four different groups attached to the chiral carbon atom are circled as shown below.

Figure 3
The structural formula for the amino acid, valine is shown in Figure 1. The chiral carbon atom in it is shown in Figure 2. The four different groups attached to the chiral carbon atom are shown in Figure 3.
(b)
Interpretation:
The structural formula for the amino acid, glutamate is to be drawn. The chiral carbon atom in it is to be identified. The four different groups attached to the chiral carbon atom are to be circled.
Concept introduction:
Amino acids are organic compounds which combine sequentially to generate a protein. They are known as the building blocks of the human body. The main elements present in amino acids are carbon, nitrogen and oxygen while the side chains attached to the chiral carbon atom contain other elements.
Answer to Problem 19.5E
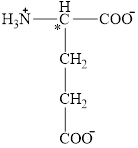
The structural formula for the amino acid, glutamate is shown below.
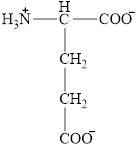
The chiral carbon atom in it is shown below.
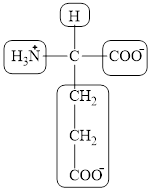
The four different groups attached to the chiral carbon atom are circled as shown below.
Explanation of Solution
Amino acids are formed by an amine and a carboxylic acid attached to a carbon atom with a characteristic side chain. The carboxylic acid in amino acids is usually in an ionic form and therefore, it is known as carboxylate group.
The structure of glutamate is given below.

Figure 4
The chiral carbon atom in it is shown below.

Figure 5
The four different groups attached to the chiral carbon atom are circled as shown below.

Figure 6
The structural formula for the amino acid, glutamate is shown in Figure 4. The chiral carbon atom in it is shown in Figure 5. The four different groups attached to the chiral carbon atom are shown in Figure 6.
(c)
Interpretation:
The structural formula for the amino acid, aspargine is to be drawn. The chiral carbon atom in it is to be identified. The four different groups attached to the chiral carbon atom are to be circled.
Concept introduction:
Amino acids are organic compounds which combine sequentially to generate a protein. They are known as the building blocks of the human body. The main elements present in amino acids are carbon, nitrogen and oxygen while the side chains attached to the chiral carbon atom contain other elements.
Answer to Problem 19.5E
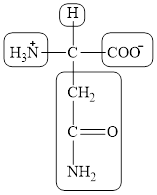
The structural formula for the amino acid, aspargine is shown below.
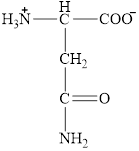
The chiral carbon atom in it is shown below.
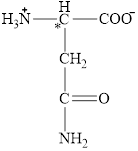
The four different groups attached to the chiral carbon atom are circled as shown below.
Explanation of Solution
Amino acids are formed by an amine and a carboxylic acid attached to a carbon atom with a characteristic side chain. The carboxylic acid in amino acids is usually in an ionic form and therefore, it is known as carboxylate group.
The structure of aspargine is given below.

Figure 7
The chiral carbon atom in it is shown below.

Figure 8
The four different groups attached to the chiral carbon atom are circled as shown below.

Figure 9
The structural formula for the amino acid, aspargine is shown in Figure 7. The chiral carbon atom in it is shown in Figure 8. The four different groups attached to the chiral carbon atom are shown in Figure 9.
(d)
Interpretation:
The structural formula for the amino acid, cysteine is to be drawn. The chiral carbon atom in it is to be identified. The four different groups attached to the chiral carbon atom are to be circled.
Concept introduction:
Amino acids are organic compounds which combine sequentially to generate a protein. They are known as the building blocks of the human body. The main elements present in amino acids are carbon, nitrogen and oxygen while the side chains attached to the chiral carbon atom contain other elements.
Answer to Problem 19.5E
The structural formula for the amino acid, cysteine is shown below.
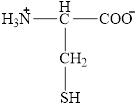
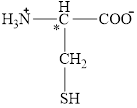
The chiral carbon atom in it is shown below.
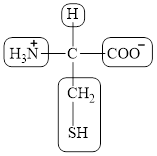
The four different groups attached to the chiral carbon atom are circled as shown below.
Explanation of Solution
Amino acids are formed by an amine and a carboxylic acid attached to a carbon atom with a characteristic side chain. The carboxylic acid in amino acids is usually in an ionic form and therefore, it is known as carboxylate group.
The structure of cysteine is given below.

Figure 10
The chiral carbon atom in it is shown below.

Figure 11
The four different groups attached to the chiral carbon atom are circled as shown below.

Figure 12
The structural formula for the amino acid, cysteine is shown in Figure 10. The chiral carbon atom in it is shown in Figure 11. The four different groups attached to the chiral carbon atom are shown in Figure 12.
Want to see more full solutions like this?
Chapter 19 Solutions
Chemistry For Today: General, Organic, And Biochemistry, Loose-leaf Version
- 2. Identify the reagents you would need to achieve the following. You may need to consider using a protecting group. HO 1. 2. 3. 4. 5. OH Br HOarrow_forwardBeF2 exists as a linear molecule. Which kind of hybrid orbitals does Be use in this compound? Use Orbital Diagrams to show how the orbitals are formed. (6)arrow_forwardPlease answer the questions and provide detailed explanations as well as a drawing to show the signals in the molecule.arrow_forward
- Propose an efficient synthesis for the following transformation: EN The transformation above can be performed with some reagent or combination of the reagents listed below. Give the necessary reagents in the correct order, as a string of letters (without spaces or punctuation, such as "EBF"). If there is more than one correct solution, provide just one answer. A. t-BuOK B. Na2Cr2O7, H2SO4, H2O C. NBS, heat F. NaCN D. MeOH E. NaOH G. MeONa H. H2O I. 1) O3; 2) DMSarrow_forwardStereochemistry Identifying the enantiomer of a simple organic molecule 1/5 Check the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of t above box under the table. Br ま HO H 0 Molecule 1 Molecule 2 Molecule 3 OH H Br H H" Br OH Br Molecule 4 Br H OH + + OH Molecule 5 Br H OH none of the above Molecule 6 Br H... OHarrow_forwardPlease answer the questions and provide detailed explanations.arrow_forward
- Question 16 0/1 pts Choose the correct option for the following cycloaddition reaction. C CF3 CF3 CF3 CF3 The reaction is suprafacial/surafacial and forbidden The reaction is antarafacial/antarafacial and forbidden The reaction is antarafacial/antarafacial and allowed The reaction is suprafacial/surafacial and allowedarrow_forward1. Give the structures of the products obtained when the following are heated. Include stereochemistry where relevant. A NO2 + NO2 B + C N=C CEN + { 2. Which compounds would you heat together in order to synthesize the following?arrow_forwardExplain how myo-inositol is different from D-chiro-inositol. use scholarly sources and please hyperlink.arrow_forward
- What is the molarisuty of a 0.396 m glucose solution if its density is 1.16 g/mL? MM glucose 180.2 /mol.arrow_forwardProvide the proper IUPAC or common name for the following compound. Dashes, commas, and spaces must be used correctly. Br ......Im OHarrow_forwardCan you please help me solve this problems. The top one is just drawing out the skeletal correct and then the bottom one is just very confusing to me and its quite small in the images. Can you enlarge it and explain it to me please. Thank You much (ME EX1) Prblm #33arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





