
Concept explainers
(a)
Interpretation:
The type of interaction present between the side chains of tyrosine and glutamine is to be stated.
Concept introduction:
The structure of proteins has four stages. These four stages are: primary, secondary, tertiary and quaternary structures. In primary structure, the amino acids are arranged to give the backbone of protein. In secondary structure, the pattern of the peptide chain is arranged in certain order. In tertiary structure, the interactions between the side chains of amino acids are considered. In quaternary structure, two or more peptide chains are linked.
Answer to Problem 19.42E
The type of interaction present between the side chains of tyrosine and glutamine is hydrogen bonding.
Explanation of Solution
The structures of tyrosine and glutamine are shown in Figure 1.
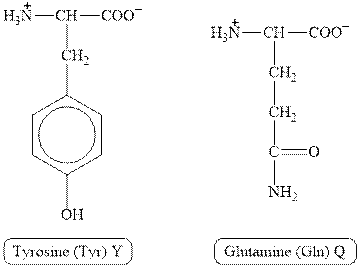
Figure 1
In tyrosine, the side chain has
Therefore, the type of interaction present between the side chains of tyrosine and glutamine is hydrogen bonding.
The type of interaction present between the side chains of tyrosine and glutamine is hydrogen bonding.
(b)
Interpretation:
The type of interaction present between the side chains of aspartate and lysine is to be stated.
Concept introduction:
The structure of proteins has four stages. These four stages are: primary, secondary, tertiary and quaternary structures. In primary structure, the amino acids are arranged to give the backbone of protein. In secondary structure, the pattern of the peptide chain is arranged in certain order. In tertiary structure, the interactions between the side chains of amino acids are considered. In quaternary structure, two or more peptide chains are linked.
Answer to Problem 19.42E
The type of interaction present between the side chains of aspartate and lysine is salt bridge.
Explanation of Solution
The structures of aspartate and lysine are shown in Figure 2.
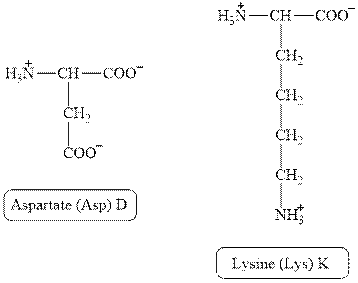
Figure 2
In aspartate, the side chain has
The type of interaction present between the side chains of aspartate and lysine is salt bridge.
(c)
Interpretation:
The type of interaction present between the side chains of leucine and isoleucine is to be stated.
Concept introduction:
The structure of proteins has four stages. These four stages are: primary, secondary, tertiary and quaternary structures. In primary structure, the amino acids are arranged to give the backbone of protein. In secondary structure, the pattern of the peptide chain is arranged in certain order. In tertiary structure, the interactions between the side chains of amino acids are considered. In quaternary structure, two or more peptide chains are linked.
Answer to Problem 19.42E
The type of interaction present between the side chains of leucine and isoleucine is hydrophobic interaction.
Explanation of Solution
The structures of leucine and isoleucine are shown in Figure 3.
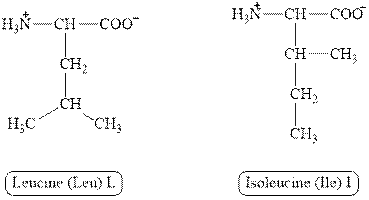
Figure 3
In leucine, the side chain has
The type of interaction present between the side chains of leucine and isoleucine is hydrophobic interaction.
(d)
Interpretation:
The type of interaction present between the side chains of phenylalanine and valine is to be stated.
Concept introduction:
The structure of proteins has four stages. These four stages are: primary, secondary, tertiary and quaternary structures. In primary structure, the amino acids are arranged to give the backbone of protein. In secondary structure, the pattern of the peptide chain is arranged in certain order. In tertiary structure, the interactions between the side chains of amino acids are considered. In quaternary structure, two or more peptide chains are linked.
Answer to Problem 19.42E
The type of interaction present between the side chains of phenylalanine and valine is hydrophobic interaction.
Explanation of Solution
The structures of phenylalanine and valine are shown in Figure 4.
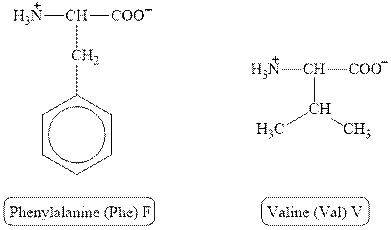
Figure 4
In phenylalanine, the side chain has
The type of interaction present between the side chains of phenylalanine and valine is hydrophobic interaction.
Want to see more full solutions like this?
Chapter 19 Solutions
Chemistry For Today: General, Organic, And Biochemistry, Loose-leaf Version
- The temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forward
- er your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward5.arrow_forward6.arrow_forward
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER





