
Concept explainers
(a)
Interpretation:
The structural formula for the amino acid, threonine is to be drawn. The chiral carbon atom in it is to be identified. The four different groups attached to the chiral carbon atom are to be circled.
Concept introduction:
Amino acids are organic compounds which combine sequentially to generate a protein. They are known as the building blocks of the human body. The main elements present in amino acids are carbon, nitrogen and oxygen while the side chains attached to the chiral carbon atom contain other elements.
Answer to Problem 19.4E
The structural formula for the amino acid, threonine is shown below.
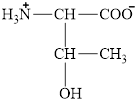
The chiral carbon atoms in it are shown below.
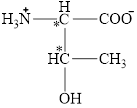
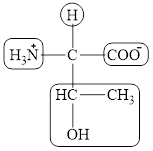
The four different groups attached to the chiral carbon atom are circled as shown below.
Explanation of Solution
Amino acids are formed by an
The structure of threonine is given below.

Figure 1
The chiral carbon atoms in it are shown below.

Figure 2
The four different groups attached to the chiral carbon atom are circled as shown below.

Figure 3
The structural formula for the amino acid, threonine is shown in Figure 1. The chiral carbon atoms in it are shown in Figure 2. The four different groups attached to the chiral carbon atom are shown in Figure 3.
(b)
Interpretation:
The structural formula for the amino acid, aspartate is to be drawn. The chiral carbon atom in it is to be identified. The four different groups attached to the chiral carbon atom are to be circled.
Concept introduction:
Amino acids are organic compounds which combine sequentially to generate a protein. They are known as the building blocks of the human body. The main elements present in amino acids are carbon, nitrogen and oxygen while the side chains attached to the chiral carbon atom contain other elements.
Answer to Problem 19.4E
The structural formula for the amino acid, aspartate is shown below.
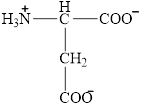
The chiral carbon atom in it is shown below.
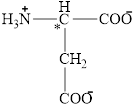
The four different groups attached to the chiral carbon atom are circled as shown below.
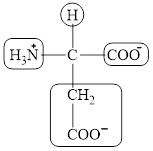
Explanation of Solution
Amino acids are formed by an amine and a carboxylic acid attached to a carbon atom with a characteristic side chain. The carboxylic acid in amino acids is usually in an ionic form and therefore, it is known as carboxylate group.
The structure of aspartate is given below.

Figure 4
The chiral carbon atom in it is shown below.

Figure 5
The four different groups attached to the chiral carbon atom are circled as shown below.

Figure 6
The structural formula for the amino acid, aspartate is shown in Figure 4. The chiral carbon atom in it is shown in Figure 5. The four different groups attached to the chiral carbon atom are shown in Figure 6.
(c)
Interpretation:
The structural formula for the amino acid, serine is to be drawn. The chiral carbon atom in it is to be identified. The four different groups attached to the chiral carbon atom are to be circled.
Concept introduction:
Amino acids are organic compounds which combine sequentially to generate a protein. They are known as the building blocks of the human body. The main elements present in amino acids are carbon, nitrogen and oxygen while the side chains attached to the chiral carbon atom contain other elements.
Answer to Problem 19.4E
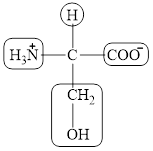
The structural formula for the amino acid, serine is shown below.
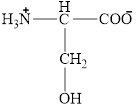
The chiral carbon atom in it is shown below.
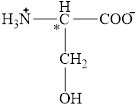
The four different groups attached to the chiral carbon atom are circled as shown below.
Explanation of Solution
Amino acids are formed by an amine and a carboxylic acid attached to a carbon atom with a characteristic side chain. The carboxylic acid in amino acids is usually in an ionic form and therefore, it is known as carboxylate group.
The structure of serine is given below.

Figure 7
The chiral carbon atom in it is shown below.

Figure 8
The four different groups attached to the chiral carbon atom are circled as shown below.

Figure 9
The structural formula for the amino acid, serine is shown in Figure 7. The chiral carbon atom in it is shown in Figure 8. The four different groups attached to the chiral carbon atom are shown in Figure 9.
(d)
Interpretation:
The structural formula for the amino acid, phenylalanine is to be drawn. The chiral carbon atom in it is to be identified. The four different groups attached to the chiral carbon atom are to be circled.
Concept introduction:
Amino acids are organic compounds which combine sequentially to generate a protein. They are known as the building blocks of the human body. The main elements present in amino acids are carbon, nitrogen and oxygen while the side chains attached to the chiral carbon atom contain other elements.
Answer to Problem 19.4E
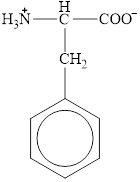
The structural formula for the amino acid, phenylalanine is shown below.
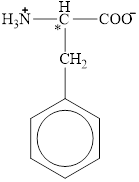
The chiral carbon atom in it is shown below.
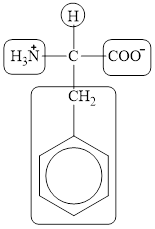
The four different groups attached to the chiral carbon atom are circled as shown below.
Explanation of Solution
Amino acids are formed by an amine and a carboxylic acid attached to a carbon atom with a characteristic side chain. The carboxylic acid in amino acids is usually in an ionic form and therefore, it is known as carboxylate group.
The structure of phenylalanine is given below.

Figure 10
The chiral carbon atom in it is shown below.

Figure 11
The four different groups attached to the chiral carbon atom are circled as shown below.

Figure 12
The structural formula for the amino acid, phenylalanine is shown in Figure 10. The chiral carbon atom in it is shown in Figure 11. The four different groups attached to the chiral carbon atom are shown in Figure 12.
Want to see more full solutions like this?
Chapter 19 Solutions
Chemistry For Today: General, Organic, And Biochemistry, Loose-leaf Version
- Draw a mechanism for the following synthetic transformation including reagents and any isolable intermediates throughout the process. Please clearly indicate bond cleavage/formation using curly arrows. MeO2Carrow_forwardCHEM 310 Quiz 8 Organic Chemistry II Due: Tuesday, April 25th, at 11:59 pm. This quiz is open textbook / open notes - but you must work alone. You cannot use the internet or the solutions manual for the book. Scan in your work and record an explanation of your mechanism. You may record this any way that you like. One way would be to start an individual Zoom meeting, start recording, "share your screen" and then talk through the problem. This will be converted to an .mp4 file that you can upload into Canvas using the "record/upload media" feature. Pyridine, benzoic acid and benzene are dissolved in ethyl acetate. Design and provide a plan / flow chart for separating and isolating each of these components. Pyridine and benzene are liquids at room temperature. Benzoic acid is a solid. You have ethyl acetate, 2M NaOH, 2M HCI and anhydrous MgSO4 available, as well as all the glassware and equipment that you used in the organic lab this year. Provide accurate acid/base reactions for any…arrow_forwardCan anyone help me solve this step by step. Thank you in advaarrow_forward
- Please draw the mechanism for this Friedel-crafts acylation reaction using arrowsarrow_forwardDraw the Fischer projection of D-fructose. Click and drag to start drawing a structure. Skip Part Check AP 14 tv SC F1 F2 80 F3 a F4 ! 2 # 3 CF F5 75 Ax MacBook Air 894 $ 5olo % Λ 6 > W F6 K F7 &arrow_forwardConsider this step in a radical reaction: Y What type of step is this? Check all that apply. Draw the products of the step on the right-hand side of the drawing area below. If more than one set of products is possible, draw any set. Also, draw the mechanism arrows on the left-hand side of the drawing area to show how this happens. ionization propagation initialization passivation none of the abovearrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,





