
Give the IUPAC name for each compound.
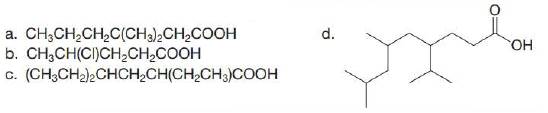
(a)
Interpretation:
The IUPAC name for the given compound is to be stated.
Concept introduction:
The chemical structures are described by IUPAC name or common names. IUPAC nameis different from common names because IUPAC nameis applied at international level and it comprises suffix, prefix, numbers and other priority rules.
Answer to Problem 19.1P
The IUPAC name of the compound is
Explanation of Solution
The numbering of carbon atoms in the given compound is shown below.
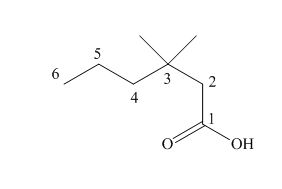
Figure 1
The given compound has a chain of six carbon atoms. The carboxylic acid is present as a functional group in the compound. Two methyl groups are present at a third carbon atom. Therefore, the name of the compound is
The IUPAC name of the given compound is
(b)
Interpretation:
The IUPAC name for the given compound is to be stated.
Concept introduction:
The chemical structures are described by IUPAC name or common names. IUPAC name is different from common names because IUPAC name is applied at international level and it comprises suffix, prefix, numbers and other priority rules.
Answer to Problem 19.1P
The IUPAC name of the compound is
Explanation of Solution
The numbering of carbon atoms in the given compound is shown below.
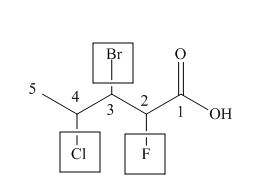
Figure 2
The given compound has a chain of five carbon atoms. The carboxylic acid is present as a functional group in the compound. Fluorine, chlorine and bromine is present at second, third and fourth carbon atoms respectively. Therefore, the name of the compound is
The IUPAC name of the compound is
(c)
Interpretation:
The IUPAC name for the given compound is to be stated.
Concept introduction:
The chemical structures are described by IUPAC name or common names. IUPAC name is different from common names because IUPAC name is applied at international level and it comprises suffix, prefix, numbers and other priority rules.
Answer to Problem 19.1P
The IUPAC name of the compound is
Explanation of Solution
The numbering of carbon atoms in the given compound is shown below.
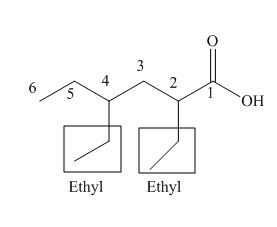
Figure 3
The given compound has a chain of six carbon atoms. The carboxylic acid is present as a functional group in the compound. Two ethyl groups are present at second and fourth carbon atoms. Therefore, the name of the compound is
The IUPAC name of the compound is
(d)
Interpretation:
The IUPAC name for the given compound is to be stated.
Concept introduction:
The chemical structures are described by IUPAC name or common names. IUPAC name is different from common names because IUPAC name is applied at international level and it comprises suffix, prefix, numbers and other priority rules.
Answer to Problem 19.1P
The IUPAC name of the compound is
Explanation of Solution
The numbering of carbon atoms in the given compound is shown below.
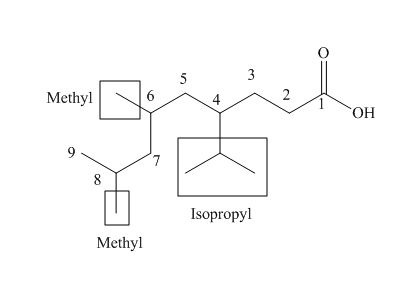
Figure 4
The given compound has a chain of nine carbon atoms. The carboxylic acid is present as a functional group in the compound. Two methyl groups are present at eighth and nine carbon atoms, while one isopropyl group is present at fourth carbon. Therefore, the name of the compound is
The IUPAC name of the compound is
Want to see more full solutions like this?
Chapter 19 Solutions
Organic Chemistry
- Part II. Identify whether the two protons in blue are homotopic, enantiopic, diasteriotopic, or heterotopic. a) HO b) Bri H HH c) d) H H H Br 0arrow_forwardNonearrow_forwardChoose the option that is decreasing from biggest to smallest. Group of answer choices: 100 m, 10000 mm, 100 cm, 100000 um, 10000000 nm 10000000 nm, 100000 um, 100 cm, 10000 mm, 100 m 10000000 nm, 100000 um, 10000 mm, 100 cm, 100 m 100 m, 100 cm, 10000 mm, 100000 um, 10000000 nmarrow_forward
- Q1. (a) Draw equations for homolytic and heterolytic cleavages of the N-H bond in NH3. Use curved arrows to show the electron movement. (b) Draw equations for homolytic and heterolytic cleavages of the N-H bond in NH4*. Use curved arrows to show the electron movement.arrow_forwardWhich is NOT the typical size of a bacteria? 1000 nm 0.001 mm 0.01 mm 1 umarrow_forwardNonearrow_forward

 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

