
Chemistry: The Central Science (14th Edition)
14th Edition
ISBN: 9780134414232
Author: Theodore E. Brown, H. Eugene LeMay, Bruce E. Bursten, Catherine Murphy, Patrick Woodward, Matthew E. Stoltzfus
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 18, Problem 8E
The first stage of treatment at the reverse osmosis plant in Carlsbad, California, is to flow the water through rock, sand, and gravel as shown here. Would this step remove particulate matter? Would this step remove dissolved salts? [Section 18.4]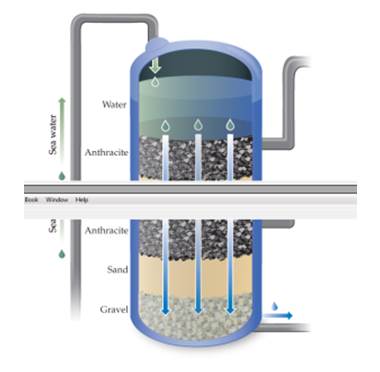
Expert Solution & Answer
Learn your wayIncludes step-by-step video

schedule02:10
Students have asked these similar questions
How many arrangements are there of 15 indistinguishable lattice gas particles distributed on:
a.V = 15 sites
b.V = 16 sites
c.V = 20 sites
For which element is the 3d subshell higher in energy than that 4s subshell?
Group of answer choices
Zr
Ca
V
Ni
ii) Molecular ion peak
:the peak corresponding to the intact molecule (with a positive charge)
What would the base peak and Molecular ion peaks when isobutane is subjected
to Mass spectrometry? Draw the structures and write the molecular weights of
the fragments.
Circle most stable cation
a) tert-butyl cation
b) Isopropyl cation c) Ethyl cation. d) Methyl cation
6. What does a loss of 15 represent in Mass spectrum?
a fragment of the molecule with a mass of 15 atomic mass units has been lost during
the ionization Process
7. Write the isotopes and their % abundance of isotopes of
i) Cl
Chapter 18 Solutions
Chemistry: The Central Science (14th Edition)
Ch. 18.1 - Prob. 18.1.1PECh. 18.1 - Prob. 18.1.2PECh. 18.1 - Prob. 18.2.1PECh. 18.1 - Practice Exercise 2 The bond energy in N2 is 941...Ch. 18.2 - Prob. 18.3.1PECh. 18.2 - Prob. 18.3.2PECh. 18 - Prob. 1DECh. 18 - Prob. 1ECh. 18 - Prob. 2ECh. 18 - The figure shows the three lowest regions of...
Ch. 18 - Prob. 4ECh. 18 - Where does the energy come from to evaporate the...Ch. 18 - Prob. 6ECh. 18 - Prob. 7ECh. 18 - The first stage of treatment at the reverse...Ch. 18 - Prob. 9ECh. 18 - Prob. 10ECh. 18 - Prob. 11ECh. 18 - How are the boundaries between the regions of the...Ch. 18 - Air pollution in the Mexico City metropolitan area...Ch. 18 - Prob. 14ECh. 18 - Prob. 15ECh. 18 - Prob. 16ECh. 18 - Prob. 17ECh. 18 - Prob. 18ECh. 18 - Distinguish between photodissociation and...Ch. 18 - Prob. 20ECh. 18 - Prob. 21ECh. 18 - Prob. 22ECh. 18 - Do the reactions involved in ozone depletion...Ch. 18 - Prob. 24ECh. 18 - Prob. 25ECh. 18 - Prob. 26ECh. 18 - Prob. 27ECh. 18 - Prob. 28ECh. 18 - Prob. 29ECh. 18 - Prob. 30ECh. 18 - Prob. 31ECh. 18 - Prob. 32ECh. 18 - Alcohol-based fuels for automobiles lead to the...Ch. 18 - Prob. 34ECh. 18 - Prob. 35ECh. 18 - Prob. 36ECh. 18 - Prob. 37ECh. 18 - Prob. 38ECh. 18 - Prob. 39ECh. 18 - Prob. 40ECh. 18 - Prob. 41ECh. 18 - Prob. 42ECh. 18 - Although there are many ions in seawater, the...Ch. 18 - The Ogallala aquifer described in the Close Look...Ch. 18 - Prob. 45ECh. 18 - Prob. 46ECh. 18 - List the common products formed when an organic...Ch. 18 - Prob. 48ECh. 18 - Prob. 49ECh. 18 - Prob. 50ECh. 18 - Prob. 51ECh. 18 - Prob. 52ECh. 18 - Prob. 53ECh. 18 - Prob. 54ECh. 18 - Prob. 55ECh. 18 - Prob. 56ECh. 18 - Prob. 57ECh. 18 - Prob. 58ECh. 18 - Prob. 59ECh. 18 - Prob. 60ECh. 18 - Prob. 61AECh. 18 - Prob. 62AECh. 18 - Prob. 63AECh. 18 - Prob. 64AECh. 18 - Prob. 65AECh. 18 - Prob. 66AECh. 18 - Prob. 67AECh. 18 - Explain, using Le Châtelier’s principle, why the...Ch. 18 - Prob. 69AECh. 18 - Prob. 70AECh. 18 - Prob. 71AECh. 18 - Prob. 72AECh. 18 - Prob. 73AECh. 18 - Prob. 74AECh. 18 - Prob. 75AECh. 18 - Prob. 76AECh. 18 - Prob. 77AECh. 18 - Prob. 78IECh. 18 - Prob. 79IECh. 18 - Prob. 80IECh. 18 - Prob. 81IECh. 18 - Prob. 82IECh. 18 - Prob. 83IECh. 18 - Prob. 84IECh. 18 - 18.85 The main reason that distillation is a...Ch. 18 - Prob. 86IECh. 18 - Prob. 87IECh. 18 - Prob. 88IECh. 18 - Prob. 89IECh. 18 - Prob. 90IECh. 18 - Prob. 91IECh. 18 - Prob. 92IE
Additional Science Textbook Solutions
Find more solutions based on key concepts
Why is petroleum jelly used in the hanging-drop procedure?
Laboratory Experiments in Microbiology (12th Edition) (What's New in Microbiology)
Another cross in Drosophila involved the recessive, X-linked genes yellow (y), white (w), and cut (ct). A yello...
Concepts of Genetics (12th Edition)
10.1 Indicate whether each of the following statements is characteristic of an acid, a base, or
both:
has a so...
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Identify each of the following reproductive barriers as prezygotic or postzygotic. a. One lilac species lives o...
Campbell Essential Biology with Physiology (5th Edition)
57. Which buffer system is the best choice to create a buffer with pH = 7.20? For the best system, calculate th...
Chemistry: A Molecular Approach (4th Edition)
Foods packed in plastic for microwaving are a. dehydrated. b. freeze-dried. c. packaged aseptically. d. commerc...
Microbiology: An Introduction
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Choose a number and match the atomic number to your element on the periodic table. For your element, write each of these features on a side of your figure. 1. Element Name and symbol 2. Family and group 3. What is it used for? 4. Sketch the Valence electron orbital 5. What ions formed. What is it's block on the periodic table. 6. Common compounds 7. Atomic number 8. Mass number 9. Number of neutrons- (show calculations) 10. Sketch the spectral display of the element 11.Properties 12. Electron configuration 13. Submit a video of a 3-meter toss in slow-moarrow_forward[In this question, there are multiple answers to type in a "fill-in-the-blank" fashion - in each case, type in a whole number.] Consider using Slater's Rules to calculate the shielding factor (S) for the last electron in silicon (Si). There will be electrons with a 0.35 S-multiplier, electrons with a 0.85 S-multiplier, and electrons with a 1.00 S-multiplier.arrow_forwardProvide the unknown for the given data.arrow_forward
- Draw the Lewis structures of two methanol (CH3OH) molecules and depict hydrogenbonding between them with dashed lines. Show all lone pairs. Provide a thorough analysis to apply concept idea into other problems.arrow_forwardSteps and explanation please.arrow_forwardHow could you distinguish between each pair of compounds below using IR? For each pair citeone bond and it’s frequency that you could use to distinguish between them. Please provide thorough analysis to apply into further problems.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

World of Chemistry
Chemistry
ISBN:9780618562763
Author:Steven S. Zumdahl
Publisher:Houghton Mifflin College Div

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER
ENVIRONMENTAL POLLUTION; Author: 7activestudio;https://www.youtube.com/watch?v=oxtMFmDTv3Q;License: Standard YouTube License, CC-BY