
Chemistry: The Central Science (14th Edition)
14th Edition
ISBN: 9780134414232
Author: Theodore E. Brown, H. Eugene LeMay, Bruce E. Bursten, Catherine Murphy, Patrick Woodward, Matthew E. Stoltzfus
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 18, Problem 8E
The first stage of treatment at the reverse osmosis plant in Carlsbad, California, is to flow the water through rock, sand, and gravel as shown here. Would this step remove particulate matter? Would this step remove dissolved salts? [Section 18.4]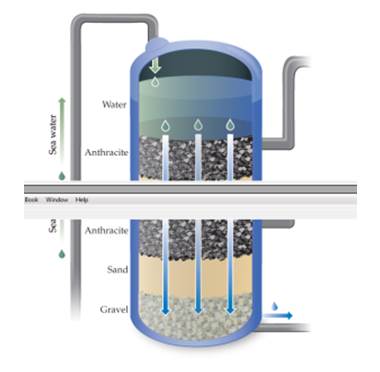
Expert Solution & Answer
Learn your wayIncludes step-by-step video

schedule02:10
Students have asked these similar questions
A mixture of 0.568 M H₂O, 0.438 M Cl₂O, and 0.710 M HClO are enclosed in a vessel at 25 °C.
H₂O(g) + C₁₂O(g) = 2 HOCl(g)
K = 0.0900 at 25°C
с
Calculate the equilibrium concentrations of each gas at 25 °C.
[H₂O]=
[C₁₂O]=
[HOCI]=
M
Σ
M
What units (if any) does the response factor (K) have? Does the response factor (K) depend upon how the concentration is expressed (e.g. molarity, ppm, ppb, etc.)?
Provide the structure, circle or draw, of the monomeric unit found in the biological polymeric
materials given below.
HO
OH
amylose
OH
OH
행
3
HO
cellulose
OH
OH
OH
Ho
HO
Chapter 18 Solutions
Chemistry: The Central Science (14th Edition)
Ch. 18.1 - Prob. 18.1.1PECh. 18.1 - Prob. 18.1.2PECh. 18.1 - Prob. 18.2.1PECh. 18.1 - Practice Exercise 2 The bond energy in N2 is 941...Ch. 18.2 - Prob. 18.3.1PECh. 18.2 - Prob. 18.3.2PECh. 18 - Prob. 1DECh. 18 - Prob. 1ECh. 18 - Prob. 2ECh. 18 - The figure shows the three lowest regions of...
Ch. 18 - Prob. 4ECh. 18 - Where does the energy come from to evaporate the...Ch. 18 - Prob. 6ECh. 18 - Prob. 7ECh. 18 - The first stage of treatment at the reverse...Ch. 18 - Prob. 9ECh. 18 - Prob. 10ECh. 18 - Prob. 11ECh. 18 - How are the boundaries between the regions of the...Ch. 18 - Air pollution in the Mexico City metropolitan area...Ch. 18 - Prob. 14ECh. 18 - Prob. 15ECh. 18 - Prob. 16ECh. 18 - Prob. 17ECh. 18 - Prob. 18ECh. 18 - Distinguish between photodissociation and...Ch. 18 - Prob. 20ECh. 18 - Prob. 21ECh. 18 - Prob. 22ECh. 18 - Do the reactions involved in ozone depletion...Ch. 18 - Prob. 24ECh. 18 - Prob. 25ECh. 18 - Prob. 26ECh. 18 - Prob. 27ECh. 18 - Prob. 28ECh. 18 - Prob. 29ECh. 18 - Prob. 30ECh. 18 - Prob. 31ECh. 18 - Prob. 32ECh. 18 - Alcohol-based fuels for automobiles lead to the...Ch. 18 - Prob. 34ECh. 18 - Prob. 35ECh. 18 - Prob. 36ECh. 18 - Prob. 37ECh. 18 - Prob. 38ECh. 18 - Prob. 39ECh. 18 - Prob. 40ECh. 18 - Prob. 41ECh. 18 - Prob. 42ECh. 18 - Although there are many ions in seawater, the...Ch. 18 - The Ogallala aquifer described in the Close Look...Ch. 18 - Prob. 45ECh. 18 - Prob. 46ECh. 18 - List the common products formed when an organic...Ch. 18 - Prob. 48ECh. 18 - Prob. 49ECh. 18 - Prob. 50ECh. 18 - Prob. 51ECh. 18 - Prob. 52ECh. 18 - Prob. 53ECh. 18 - Prob. 54ECh. 18 - Prob. 55ECh. 18 - Prob. 56ECh. 18 - Prob. 57ECh. 18 - Prob. 58ECh. 18 - Prob. 59ECh. 18 - Prob. 60ECh. 18 - Prob. 61AECh. 18 - Prob. 62AECh. 18 - Prob. 63AECh. 18 - Prob. 64AECh. 18 - Prob. 65AECh. 18 - Prob. 66AECh. 18 - Prob. 67AECh. 18 - Explain, using Le Châtelier’s principle, why the...Ch. 18 - Prob. 69AECh. 18 - Prob. 70AECh. 18 - Prob. 71AECh. 18 - Prob. 72AECh. 18 - Prob. 73AECh. 18 - Prob. 74AECh. 18 - Prob. 75AECh. 18 - Prob. 76AECh. 18 - Prob. 77AECh. 18 - Prob. 78IECh. 18 - Prob. 79IECh. 18 - Prob. 80IECh. 18 - Prob. 81IECh. 18 - Prob. 82IECh. 18 - Prob. 83IECh. 18 - Prob. 84IECh. 18 - 18.85 The main reason that distillation is a...Ch. 18 - Prob. 86IECh. 18 - Prob. 87IECh. 18 - Prob. 88IECh. 18 - Prob. 89IECh. 18 - Prob. 90IECh. 18 - Prob. 91IECh. 18 - Prob. 92IE
Additional Science Textbook Solutions
Find more solutions based on key concepts
Why is petroleum jelly used in the hanging-drop procedure?
Laboratory Experiments in Microbiology (12th Edition) (What's New in Microbiology)
Another cross in Drosophila involved the recessive, X-linked genes yellow (y), white (w), and cut (ct). A yello...
Concepts of Genetics (12th Edition)
10.1 Indicate whether each of the following statements is characteristic of an acid, a base, or
both:
has a so...
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Identify each of the following reproductive barriers as prezygotic or postzygotic. a. One lilac species lives o...
Campbell Essential Biology with Physiology (5th Edition)
57. Which buffer system is the best choice to create a buffer with pH = 7.20? For the best system, calculate th...
Chemistry: A Molecular Approach (4th Edition)
Foods packed in plastic for microwaving are a. dehydrated. b. freeze-dried. c. packaged aseptically. d. commerc...
Microbiology: An Introduction
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- OA. For the structure shown, rank the bond lengths (labeled a, b and c) from shortest to longest. Place your answer in the box. Only the answer in the box will be graded. (2 points) H -CH3 THe b Нarrow_forwardDon't used hand raitingarrow_forwardQuizzes - Gen Organic & Biological Che... ☆ myd21.lcc.edu + O G screenshot on mac - Google Search savings hulu youtube google disney+ HBO zlib Homework Hel...s | bartleby cell bio book Yuzu Reader: Chemistry G periodic table - Google Search b Home | bartleby 0:33:26 remaining CHEM 120 Chapter 5_Quiz 3 Page 1: 1 > 2 > 3 > 6 ¦ 5 > 4 > 7 ¦ 1 1 10 8 ¦ 9 a ¦ -- Quiz Information silicon-27 A doctor gives a patient 0.01 mC i of beta radiation. How many beta particles would the patient receive in I minute? (1 Ci = 3.7 x 10 10 d/s) Question 5 (1 point) Saved Listen 2.22 x 107 222 x 108 3.7 x 108 2.22 x 108 none of the above Question 6 (1 point) Listen The recommended dosage of 1-131 for a test is 4.2 μCi per kg of body mass. How many millicuries should be given to a 55 kg patient? (1 mCi = 1000 μСi)? 230 mCiarrow_forward
- Q4: Rank the relative nucleophilicity of halide ions in water solution and DMF solution, respectively. F CI Br | Q5: Determine which of the substrates will and will not react with NaSCH3 in an SN2 reaction to have a reasonable yield of product. NH2 Br Br Br .OH Brarrow_forwardClassify each molecule as optically active or inactive. Determine the configuration at each H соон Chirality center OH 애 He OH H3C Ноос H H COOH A K B.arrow_forwardQ1: Rank the relative nucleophilicity of the following species in ethanol. CH3O¯, CH3OH, CH3COO, CH3COOH, CH3S Q2: Group these solvents into either protic solvents or aprotic solvents. Acetonitrile (CH3CN), H₂O, Acetic acid (CH3COOH), Acetone (CH3COCH3), CH3CH2OH, DMSO (CH3SOCH3), DMF (HCON(CH3)2), CH3OHarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

World of Chemistry
Chemistry
ISBN:9780618562763
Author:Steven S. Zumdahl
Publisher:Houghton Mifflin College Div

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER
ENVIRONMENTAL POLLUTION; Author: 7activestudio;https://www.youtube.com/watch?v=oxtMFmDTv3Q;License: Standard YouTube License, CC-BY