
(a)
Interpretation:
The given amine needs to be labeled as 10, 20 or 30.
Concept introduction:
Answer to Problem 74P
A tertiary (3o) amine.
Explanation of Solution
Amines are derivatives which are derived from ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. They can be called as alkylamines and arylamines.
Whether an amine is primary (1o), secondary (2o) or tertiary (3o) depends on the number of hydrogen atoms replaced by an alkyl or aryl group in ammonia. The amine is a primary amine if one hydrogen atom is replaced, it is a secondary amine if 2 hydrogen atoms are replaced and if three hydrogen atoms are replaced it is known as a tertiary amine.
The given compound is as follows:
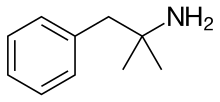
Here, only one hydrogen atom is replaced and therefore this amine called as a primary (1o) amine.
(b)
Interpretation:
The molecular shape around each atom of phentermine needs to be determined.
Concept introduction:
Molecular geometry is named as molecular shape and it is the three-dimensional arrangement of atoms within a molecule of the environment. Atoms arrange in space with an exact shape to minimize the repulsion. This can be determined using VSEPR theory.
Answer to Problem 74P
Carbons of benzene molecule;trigonal planar.
Carbon of −CH2 / -C(CH3)2NH2 CH2-/-CH3;tetrahedral.
Nitrogen of NH2;trigonal pyramidal.
Explanation of Solution
VSEPR or Valence Shell Electron Pair Repulsion theory is a model used to determine the geometry of molecules considering minimum electrostatic repulsion between the valence electrons of central atom in the molecule.
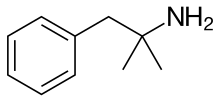
Carbons of benzene molecule;
Number of bonds= 3
Number of lone pairs = 0
Therefore, the shape is trigonal planar.
Carbon of −CH2/ -C(CH3)2NH2CH2-/-CH3;
Number of bonds= 4
Number of lone pairs = 0
Therefore, the shape is tetrahedral.
Nitrogen of NH2;
Number of bonds= 3
Number of lone pairs = 1
Therefore, the shape is trigonal pyramidal.
(c)
Interpretation:
The constitutional isomer containing a primary amine needs to be determined.
Concept introduction:
Constitutional isomers are called as compounds that have the unique molecular formula and different structural connectivity. To determine whether two molecules are constitutional isomer, the number of each atom needs to be counted in both molecules and see how the atoms are arranged.
Answer to Problem 74P
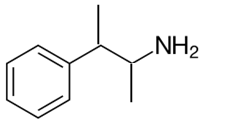
Explanation of Solution
Whether an amine is primary (1o), secondary (2o) or tertiary (3o) depends on the number of hydrogen atoms replaced by an alkyl or aryl group in ammonia. The amine is a primary amine if one hydrogen atom is replaced, it is a secondary amine if 2 hydrogen atoms are replaced and if three hydrogen atoms are replaced it is known as a tertiary amine.

Here all one hydrogen atom is replaced and therefore this amine called a primary amine (1o).
(d)
Interpretation:
The constitutional isomer that contains a secondary amine needs to be determined.
Concept introduction:
Constitutional isomers are called as compounds that have the unique molecular formula and different structural connectivity. To determine whether two molecules are constitutional isomer, the number of each atom needs to be counted in both molecules and see how the atoms are arranged.
Answer to Problem 74P
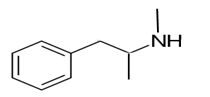
Explanation of Solution
Whether an amine is primary (1o), secondary (2o) or tertiary (3o) depends on the number of hydrogen atoms replaced by an alkyl or aryl group in ammonia. The amine is a primary amine if one hydrogen atom is replaced, it is a secondary amine if 2 hydrogen atoms are replaced and if three hydrogen atoms are replaced it is known as a tertiary amine.

Here all 3 hydrogen atoms are replaced and therefore this amine called as a secondary amine (2o).
(e)
Interpretation:
The structure of phentermine hydrobromide molecule needs to be drawn.
Concept introduction:
Phentermine hydrobromide can stimulate the central nervous system and increases the blood pressure and heart rate which decreases the appetite. It is taken along with diet and exercise in order to reduce obesity.
Answer to Problem 74P
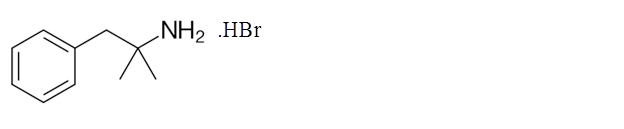
Explanation of Solution
Phentermine hydrobromide can stimulate the central nervous system and increases the blood pressure and heart rate which decreases the appetite. It is taken along with the diet and exercise in order to reduce the obesity.
Its molecular formula is C17H22BrN. It has the following structure.
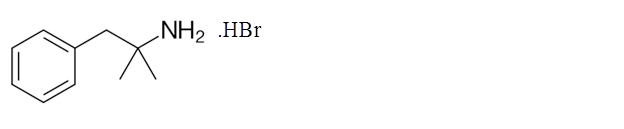
(f)
Interpretation:
The products formed if phentermine is treated with benzoic acid needs to be determined.
Concept introduction:
Amines are derivatives which are derived from ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. They can be called as alkylamines and arylamines.
Answer to Problem 74P
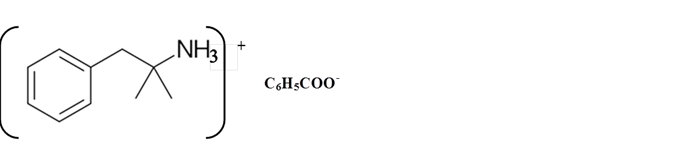
Explanation of Solution
Amines are derivatives which are derived from ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. They can be called as alkylamines and arylamines.
Whether an amine is primary (1o), secondary (2o) or tertiary (3o) depends on the number of hydrogen atoms replaced by an alkyl or aryl group in ammonia. The amine is a primary amine if one hydrogen atom is replaced, it is a secondary amine if 2 hydrogen atoms are replaced and if three hydrogen atoms are replaced it is known as a tertiary amine.
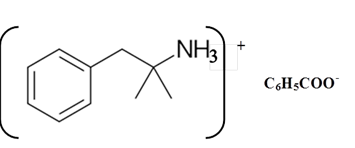
Benzphetamine is a tertiary ammine. It has basic properties and it accepts protons from acids. Acetic acid is donated a proton to this amine and formed below the salt.
Want to see more full solutions like this?
Chapter 18 Solutions
GENERAL,ORGANIC, & BIOLOGICAL CHEM-ACCES
- true or false, given that a 20.00 mL sample of NaOH took 24.15 mL of 0.141 M HCI to reach the endpoint in a titration, the concentration of the NaOH is 1.17 M.arrow_forwardin the bromothymol blue experiment, pKa was measured. A closely related compound has a Ka of 2.10 x 10-5. What is the pKa?a) 7.1b) 4.7c) 2.0arrow_forwardcalculate the equilibrium concentration of H2 given that K= 0.017 at a constant temperature for this reaction. The inital concentration of HBr is 0.050 M.2HBr(g) ↔ H2(g) + Br2(g)a) 4.48 x 10-2 M b) 5.17 x 10-3 Mc) 1.03 x 10-2 Md) 1.70 x 10-2 Marrow_forward
- true or falsegiven these two equilibria with their equilibrium constants:H2(g) + CI2(l) ↔ 2HCI(g) K= 0.006 CI2(l) ↔ CI2(g) K= 0.30The equilibrium contstant for the following reaction is 1.8H2(g) + CI2 ↔ 2HCI(g)arrow_forwardI2(g) + CI2(g) ↔ 2ICIK for this reaction is 81.9. Find the equilibrium concentration of I2 if the inital concentration of I2 and CI2 are 0.010 Marrow_forwardtrue or false,the equilibrium constant for this reaction is 0.50.PCI5(g) ↔ PCI3(g) + CI2(g)Based on the above, the equilibrium constant for the following reaction is 0.25.2PCI5(g) ↔. 2PCI3(g) + 2CI2(g)arrow_forward
- true or false, using the following equilibrium, if carbon dioxide is added the equilibrium will shift toward the productsC(s) + CO2(g) ↔ 2CO(g)arrow_forward2S2O2/3- (aq) + I2 (aq) ---> S4O2/6- (aq) +2I- (aq) Experiment I2 (M) S2O3- (M) Initital Rate (M/s) 1 0.01 0.01 0.0004 2 0.01 0.02 0.0004 3 0.02 0.01 0.0008 Calculate the overall order for this reaction using the table data a) 3b) 0c) 2d) 1arrow_forwardthe decomposition of N2O5 is the first order with a half-life of 1.98 minutes. If the inital concentration of N2O5 is 0.200 M, what is the concentration after 6 minutes?a) 0.612 Mb) 0.035 Mc) 0.024 Md) 0.100 Marrow_forward
- 20.00 mL of 0.150 M HCI is titrated with 0.075 M NaOH. What volume of NaOH is needed?a) 50 mLb) 20 mLc) 40 mLd) 26.66 mLarrow_forward20.00 mL of 0.150 M NaOH is titrated with 37.75 mL of HCI. What is the molarity of the HCI?a) 0.150 Mb) 0.079 Mc) 0.025 Md) 0.050 Marrow_forwardin the following reaction, the OH- acts as which of these?NO2- (aq) + H2O (l) ⇌ OH- (aq) + HNO2 (aq)a) not a weak acidb) basec) acidarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

