
Student Workbook for Physics for Scientists and Engineers: A Strategic Approach, Vol 1. (Chs 1-21)
4th Edition
ISBN: 9780134110646
Author: Randall D. Knight (Professor Emeritus)
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 18, Problem 73EAP
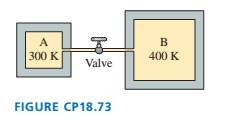
Containers A and B in FIGURE CP18.73 hold the same gas. The
volume of B is four times the volume of A. The two containers
are connected by a thin tube (negligible volume) and a valve that
is closed. The gas in A is at 300 K and pressure of 1.0 X 105 Pa.
"The gas in B is at 400 K and pressure of 5.0 X 105 Pa. Heaters
will maintain the temperatures of A and B even after the valve is
opened. After the valve is opened, gas will flow one way or the
other until A and B have equal pressure. What is this final pressure?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
A cylinder contains 3.0 L of oxygen at 300 K and 2.4 atm. The gas is heated, causing a piston in the cylinder to move outward. The heating causes the temperature to rise to 600 K and the volume of the cylinder to increase to 9.0 L. What is the final gas pressure?
A high-pressure gas cylinder contains 80.0 L of toxic gas at a pressure of 1.20 107 N/m2 and a temperature of 12.0°C. Its valve leaks after the cylinder is dropped. The cylinder is cooled to dry ice temperatures (−78.5°C), to reduce the leak rate and pressure so that it can be safely repaired. what is the final pressure in the tank, assuming a negligible amount of gas leaks while being cooled and that there is no phase change? What is the final pressure if one-tenth of the gas escapes? To what temperature must the tank be cooled to reduce the pressure to 1.00 atm (assuming the gas does not change phase and that there is no leakage during cooling)?
Problem 6: Suppose a 26.5°C car tire contains 3.5 mol of gas in a 32.5 L volume.
Part (a) What is the gauge pressure, in atmospheres, in the car tire?
P21=1.65 vCorrect!
Part (b) What will the gauge pressure be if you add a quantity of gas that had a volume of 2.00 L when it was at atmospheric pressure and the
same temperature as the tire? Assume the temperature returns to 26.5°C and the volume remains constant.
P22= 1.5|
g,2
Chapter 18 Solutions
Student Workbook for Physics for Scientists and Engineers: A Strategic Approach, Vol 1. (Chs 1-21)
Ch. 18 - Prob. 1CQCh. 18 - Prob. 2CQCh. 18 - Prob. 3CQCh. 18 - Prob. 4CQCh. 18 - Prob. 5CQCh. 18 - Prob. 6CQCh. 18 - Prob. 7CQCh. 18 - Prob. 8CQCh. 18 - Prob. 9CQCh. 18 - A gas undergoes the process shown in FIGURE...
Ch. 18 - Prob. 11CQCh. 18 - Prob. 12CQCh. 18 - Prob. 1EAPCh. 18 - Prob. 2EAPCh. 18 - What is the diameter of a copper sphere that has...Ch. 18 - Prob. 4EAPCh. 18 - Prob. 5EAPCh. 18 - How many atoms are in a 2.0 cm × 2.0 cm × 2.0 cm...Ch. 18 - Prob. 7EAPCh. 18 - An element in its solid phase has mass density...Ch. 18 - .0 mol of gold is shaped into a sphere. What is...Ch. 18 - What volume of aluminum has the same number of...Ch. 18 - Prob. 11EAPCh. 18 - Prob. 12EAPCh. 18 - Prob. 13EAPCh. 18 - A concrete bridge is built of 325-cm-long concrete...Ch. 18 - A surveyor has a steel measuring tape that is...Ch. 18 - Two students each build a piece of scientific...Ch. 18 - Prob. 17EAPCh. 18 -
18. What is the temperature in °F and the...Ch. 18 - Prob. 19EAPCh. 18 - .0 mol of gas at a temperature of -120°C fills a...Ch. 18 - Prob. 21EAPCh. 18 - Prob. 22EAPCh. 18 - Prob. 23EAPCh. 18 - Prob. 24EAPCh. 18 - Prob. 25EAPCh. 18 - Prob. 26EAPCh. 18 - Prob. 27EAPCh. 18 - Prob. 28EAPCh. 18 - A rigid, hollow sphere is submerged in boiling...Ch. 18 -
30. A rigid container holds hydrogen gas at a...Ch. 18 - Prob. 31EAPCh. 18 - Prob. 32EAPCh. 18 - Prob. 33EAPCh. 18 - Prob. 34EAPCh. 18 - Prob. 35EAPCh. 18 - Prob. 36EAPCh. 18 - Prob. 37EAPCh. 18 - .0050 mol of gas undergoes the process 1 2 3...Ch. 18 - Prob. 39EAPCh. 18 - Prob. 40EAPCh. 18 - Prob. 41EAPCh. 18 - Prob. 42EAPCh. 18 - Prob. 43EAPCh. 18 - A 15°C, 2.0-cm-diameter aluminum bar just barely...Ch. 18 - Prob. 45EAPCh. 18 - Prob. 46EAPCh. 18 - Prob. 47EAPCh. 18 - Prob. 48EAPCh. 18 - Prob. 49EAPCh. 18 - The 3.0-m-long pipe in FIGURE P18.50 is closed at...Ch. 18 - Prob. 51EAPCh. 18 - An electric generating plant boils water to...Ch. 18 - Prob. 53EAPCh. 18 - The air temperature and pressure in a laboratory...Ch. 18 - Prob. 55EAPCh. 18 - The mercury manometer shown in FIGURE P18.56 is...Ch. 18 - Prob. 57EAPCh. 18 - The 50 kg circular piston shown in FIGURE P18.58...Ch. 18 - Prob. 59EAPCh. 18 - .0 g of helium gas follows the process 1? 2 ?3...Ch. 18 - Prob. 61EAPCh. 18 - 62. FIGURE P18.62 shows two different processes...Ch. 18 - Prob. 63EAPCh. 18 - Prob. 64EAPCh. 18 - Prob. 65EAPCh. 18 - Prob. 66EAPCh. 18 - Prob. 67EAPCh. 18 - Prob. 68EAPCh. 18 - Prob. 69EAPCh. 18 - Prob. 70EAPCh. 18 - Prob. 71EAPCh. 18 - The cylinder in FIGURE CP18.72 has a moveable...Ch. 18 - Containers A and B in FIGURE CP18.73 hold the same...Ch. 18 - Prob. 74EAP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Cylinder A contains oxygen (O2) gas, and cylinder B contains nitrogen (N2) gas. If the molecules in the two cylinders have the same rms speeds, which of the following statements is false? (a) The two gases haw different temperatures. (b) The temperature of cylinder B is less than the temperature of cylinder A. (c) The temperature of cylinder B is greater than the temperature of cylinder A. (d) The average kinetic energy of the nitrogen molecules is less than the average kinetic energy of the oxygen molecules.arrow_forwardA sample of a monatomic ideal gas occupies 5.00 L at atmospheric pressure and 300 K (point A in Fig. P21.65). It is warmed at constant volume to 3.00 atm (point B). Then it is allowed to expand isothermally to 1.00 atm (point C) and at last compressed isobarically to its original state, (a) Find the number of moles in the sample. Find (b) the temperature at point B, (c) the temperature at point C, and (d) the volume at point C. (e) Now consider the processes A B, B C, and C A. Describe how to carry out each process experimentally, (f) Find Q, W, and Eint for each of the processes, (g) For the whole cycle A B C A, find Q, W, and Eint.arrow_forwardOn a hot summer day, the density of air at atmospheric pressure at 35.0C is 1.1455 kg/m3. a. What is the number of moles contained in 1.00 m3 of an ideal gas at this temperature and pressure? b. Avogadros number of air molecules has a mass of 2.85 102 kg. What is the mass of 1.00 m3 of air? c. Does the value calculated in part (b) agree with the stated density of air at this temperature?arrow_forward
- A gas is at 200 K. If we wish to double the rms speed of the molecules of the gas, to what value must we raise its temperature? (a) 283 K (b) 400 K (c) 566 K (d) 800 K (e) 1 130 Karrow_forwardA certain ideal gas has a molar specific heat of Cv = 72R. A 2.00-mol sample of the gas always starts at pressure 1.00 105 Pa and temperature 300 K. For each of the following processes, determine (a) the final pressure, (b) the final volume, (c) the final temperature, (d) the change in internal energy of the gas, (e) the energy added to the gas by heat, and (f) the work done on the gas. (i) The gas is heated at constant pressure to 400 K. (ii) The gas is heated at constant volume to 400 K. (iii) The gas is compressed at constant temperature to 1.20 105 Pa. (iv) The gas is compressed adiabatically to 1.20 105 Pa.arrow_forward(a) Show that the density of an ideal gas occupying a volume V is given by = PM/KT, where M is the molar mass. (b) Determine the density of oxygen gas at atmospheric pressure and 20.0C.arrow_forward
- The pressure gauge on a cylinder of gas registers the gauge pressure, which is the difference between the interior pressure and the exterior pressure P0. Lets call the gauge pressure Pg. When the cylinder is full, the mass of the gas in it is mi at a gauge pressure of Pgi. Assuming the temperature of the cylinder remains constant, show that the mass of the gas remaining in the cylinder when the pressure reading is Pgf is given by mf=mi(Pgf+P0Pgi+P0)arrow_forwardA cylinder with a piston holds 0.50 m3 of oxygen at an absolute pressure of 4.0 atm. The piston is pulled outward, increasing the volume of the gas until the pressure drops to 1.0 atm. If the temperature stays constant, what new volume does the gas occupy? (a) 1.0 m3 (b) 1.5 m3 (c) 2.0 m3 (d) 0.12 m3 (e) 2.5 m3arrow_forwardIn Figure P19.22, the change in internal energy of a gas that is taken from A to C along the blue path is +800 J. The work done on the gas along the red path ABC is 500 J. (a) How much energy must be added to the system by heat as it goes from A through B to C? (b) If the pressure at point A is five times that of point C, what is the work done on the system in going from C to D? Figure P19.22 (c) What is the energy exchanged with the surroundings by heat as the gas goes from C to A along the green path? (d) If the change in internal energy in going from point D to point A is +500 J, how much energy must be added to the system by heat as it goes from point C to point D?arrow_forward
- A 40.0-g projectile is launched by the expansion of hot gas in an arrangement shown in Figure P12.4a. The cross sectional area of the launch tube is 1.0 cm2, and the length that the projectile travels down the tube after starting from rest is 52 cm. As the gas expands, the pressure varies as shown in Figure P12.4b. The values for the initial pressure and volume are P1 = 11 105 Pa and Vi = 8.0 cm3 while the final values are Pf = 1.0 105 Pa and Vf = 8.0 cm3. Friction between the projectile and the launch tube is negligible, (a) If the projectile is launched into a vacuum, what is the speed of the projectile as it leaves the launch tube? (b) If instead the projectile is launched into air at a pressure of 1.0 105 Pa. what fraction of the work done by the expanding gas in the tube is spent by the projectile pushing air out of the way as it proceeds down tile tube?arrow_forwardSuppose 26.0 g of neon gas are stored in a tank at a temperature of 152C. (a) What is the temperature of the gas on the Kelvin scale? (See Section 10.2.) (b) How many moles of gas are in the tank? (See Section 10.4.) (c) What is the internal energy of the gas? (See Section 10.5.)arrow_forwardWhy is the following situation impossible? An ideal gas undergoes a process with the following parameters: Q = 10.0 J, W = 12.0 J, and T = 2.00C.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning
Thermodynamics: Crash Course Physics #23; Author: Crash Course;https://www.youtube.com/watch?v=4i1MUWJoI0U;License: Standard YouTube License, CC-BY