
Student Workbook for Physics for Scientists and Engineers: A Strategic Approach, Vol 1. (Chs 1-21)
4th Edition
ISBN: 9780134110646
Author: Randall D. Knight (Professor Emeritus)
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 18, Problem 58EAP
The 50 kg circular piston shown
in FIGURE P18.58 floats on 0. 12 mol
of compressed air.
a. What is the piston height h if
the temperature is 30oC?
b. How far does the piston move
if the temperature is increased
by 100oC?
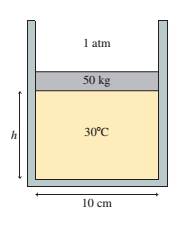
FIGURE P18.58
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
A fluid with density 263 kg/m3 flows through a pipe of varying diameter and height. At location 1 the flow speed is 13.5 m/s and the diameter of the pipe is 7.4 cm down to location 2 the pipe diameter is 16.9 cm. Location 1 is 6.3 meters higher than location 2.
What is the difference in pressure P2 - P1?
Using units in Pascals and use g = 9.81 m/s2.
The kitchen had a temperature 46 degrees Fahrenheit and was converted it to Kelvin. What is the correct number for this temperature (46 F) on the Kelvin scale?
Water is traveling at a speed of 0.65 m/s through a pipe with a cross-section radius of 0.23 meters. The water enters a section of pipe that has a smaller radius, only 0.11 meters. What is the speed of the water traveling in this narrower section of pipe?
Chapter 18 Solutions
Student Workbook for Physics for Scientists and Engineers: A Strategic Approach, Vol 1. (Chs 1-21)
Ch. 18 - Prob. 1CQCh. 18 - Prob. 2CQCh. 18 - Prob. 3CQCh. 18 - Prob. 4CQCh. 18 - Prob. 5CQCh. 18 - Prob. 6CQCh. 18 - Prob. 7CQCh. 18 - Prob. 8CQCh. 18 - Prob. 9CQCh. 18 - A gas undergoes the process shown in FIGURE...
Ch. 18 - Prob. 11CQCh. 18 - Prob. 12CQCh. 18 - Prob. 1EAPCh. 18 - Prob. 2EAPCh. 18 - What is the diameter of a copper sphere that has...Ch. 18 - Prob. 4EAPCh. 18 - Prob. 5EAPCh. 18 - How many atoms are in a 2.0 cm × 2.0 cm × 2.0 cm...Ch. 18 - Prob. 7EAPCh. 18 - An element in its solid phase has mass density...Ch. 18 - .0 mol of gold is shaped into a sphere. What is...Ch. 18 - What volume of aluminum has the same number of...Ch. 18 - Prob. 11EAPCh. 18 - Prob. 12EAPCh. 18 - Prob. 13EAPCh. 18 - A concrete bridge is built of 325-cm-long concrete...Ch. 18 - A surveyor has a steel measuring tape that is...Ch. 18 - Two students each build a piece of scientific...Ch. 18 - Prob. 17EAPCh. 18 -
18. What is the temperature in °F and the...Ch. 18 - Prob. 19EAPCh. 18 - .0 mol of gas at a temperature of -120°C fills a...Ch. 18 - Prob. 21EAPCh. 18 - Prob. 22EAPCh. 18 - Prob. 23EAPCh. 18 - Prob. 24EAPCh. 18 - Prob. 25EAPCh. 18 - Prob. 26EAPCh. 18 - Prob. 27EAPCh. 18 - Prob. 28EAPCh. 18 - A rigid, hollow sphere is submerged in boiling...Ch. 18 -
30. A rigid container holds hydrogen gas at a...Ch. 18 - Prob. 31EAPCh. 18 - Prob. 32EAPCh. 18 - Prob. 33EAPCh. 18 - Prob. 34EAPCh. 18 - Prob. 35EAPCh. 18 - Prob. 36EAPCh. 18 - Prob. 37EAPCh. 18 - .0050 mol of gas undergoes the process 1 2 3...Ch. 18 - Prob. 39EAPCh. 18 - Prob. 40EAPCh. 18 - Prob. 41EAPCh. 18 - Prob. 42EAPCh. 18 - Prob. 43EAPCh. 18 - A 15°C, 2.0-cm-diameter aluminum bar just barely...Ch. 18 - Prob. 45EAPCh. 18 - Prob. 46EAPCh. 18 - Prob. 47EAPCh. 18 - Prob. 48EAPCh. 18 - Prob. 49EAPCh. 18 - The 3.0-m-long pipe in FIGURE P18.50 is closed at...Ch. 18 - Prob. 51EAPCh. 18 - An electric generating plant boils water to...Ch. 18 - Prob. 53EAPCh. 18 - The air temperature and pressure in a laboratory...Ch. 18 - Prob. 55EAPCh. 18 - The mercury manometer shown in FIGURE P18.56 is...Ch. 18 - Prob. 57EAPCh. 18 - The 50 kg circular piston shown in FIGURE P18.58...Ch. 18 - Prob. 59EAPCh. 18 - .0 g of helium gas follows the process 1? 2 ?3...Ch. 18 - Prob. 61EAPCh. 18 - 62. FIGURE P18.62 shows two different processes...Ch. 18 - Prob. 63EAPCh. 18 - Prob. 64EAPCh. 18 - Prob. 65EAPCh. 18 - Prob. 66EAPCh. 18 - Prob. 67EAPCh. 18 - Prob. 68EAPCh. 18 - Prob. 69EAPCh. 18 - Prob. 70EAPCh. 18 - Prob. 71EAPCh. 18 - The cylinder in FIGURE CP18.72 has a moveable...Ch. 18 - Containers A and B in FIGURE CP18.73 hold the same...Ch. 18 - Prob. 74EAP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- A particular water pipe has a radius of 0.28 meters. If the pipe is completely filled with water, moving with average velocity 0.45 m/s, what is the flow rate of water through the pipe with units of cubic meters of water per second?arrow_forwardWater is flowing through a horizontal pipe with two segments. In one segment, the water flows at a speed v1 = 4.52 m/s. In the second segment the speed of the water is v2 = 2.38 m/s. Based on Bernoulli's Principle, what is the difference in pressure (P2 - P1) between the two segments? Assume that the density of the water is 997 kg/m3 and give your answer as the number of Pascals (i.e. N/m2).arrow_forwardWater from the faucet is supplied to the hose at a rate of 0.00057 m3/s. At what speed (number of meters per second) does the water exit the nozzle if the cross sectional area of the narrow nozzle is 2.1 x 10-6 m2?arrow_forward
- Jason Fruits/Indiana University Research Communications Silver/ silver oxide Zinc zinc/oxidearrow_forwardCar P moves to the west with constant speed v0 along a straight road. Car Q starts from rest at instant 1, and moves to the west with increasing speed. At instant 5, car Q has speed w0 relative to the road (w0 < v0). Instants 1-5 are separated by equal time intervals. At instant 3, cars P and Q are adjacent to one another (i.e., they have the same position). In the reference frame o f the road, at instant 3 i s the speed o f car Q greater than, less than, or equal to the speed of car P? Explain.arrow_forwardCar P moves to the west with constant speed v0 along a straight road. Car Q starts from rest at instant 1, and moves to the west with increasing speed. At instant 5, car Q has speed w0 relative to the road (w0 < v0). Instants 1-5 are separated by equal time intervals.arrow_forward
- Car P moves to the west with constant speed v0 along a straight road. Car Q starts from rest at instant 1, and moves to the west with increasing speed. At instant 5, car Q has speed w0 relative to the road (w0 < v0). Instants 1-5 are separated by equal time intervals. Sketch and label a vector diagram illustrating the Galilean transformation of velocities that relates velocity of car P relative to the road, velocity of car Q relative to road, and velocity of car Q relative to car P at instant 3. In the frame of car P, at instant 3 is car Q moving to the west, moving to the east, or at rest? Explain.arrow_forwardJust 5 and 6 don't mind 7arrow_forwardIn an electron gun, electrons are accelerated through a region with an electric field of magnitude 1.5 × 104 N/C for a distance of 2.5 cm. If the electrons start from rest, how fast are they moving after traversing the gun?arrow_forward
- Please solve and answer this problem correctly please. Thank you!!arrow_forwardPlease solve and answer this problem correctly please. Thank you!!arrow_forwarda) Use the node-voltage method to find v1, v2, and v3 in the circuit in Fig. P4.14. b) How much power does the 40 V voltage source deliver to the circuit? Figure P4.14 302 202 w w + + + 40 V V1 80 Ω 02 ΣΑΩ 28 A V3 + w w 102 202arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
 Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning

College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning


Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning
Thermodynamics: Crash Course Physics #23; Author: Crash Course;https://www.youtube.com/watch?v=4i1MUWJoI0U;License: Standard YouTube License, CC-BY