
EBK ORGANIC CHEMISTRY
8th Edition
ISBN: 8220102744127
Author: Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 18, Problem 66P
Use the four compounds shown below to answer the following questions:
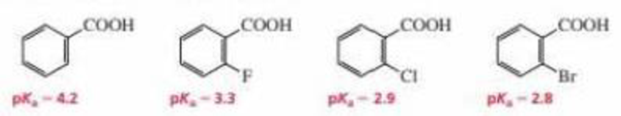
- a. Why are the ortho-halo-substituted benzoic acids stronger acids than benzoic acid?
- b. Why is o-fluorobenzoic acid the weakest of the ortho-halo-substituted benzoic acids?
- c. Why do o-chlorobenzoic acid and o-bromobenzoic acid have similar pKa values?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
2CIO2 + 20H-1 CIO31 + CIO2 + H2O
Experiment
[CIO2], M
[OH-1], M
1
0.0500
0.100
23
2
0.100
0.100
3
0.100
0.0500
Initial Rate, M/s
0.0575
0.230
0.115
...
Given this date, calculate the overall order of this reaction.
2
3
.(be)_[Ɔ+(be)_OI ← (b²)_IƆO+ (be)_I
Experiment
[1-] M
0.005
[OCI-]
0.005
Initial Rate M/min
0.000275
0.0025
0.005
0.000138
0.0025
0.0025
0.000069
4
0.0025
0.0025
0.000140
Calculate the rate constant of this reaction using the table data.
1
2
3
4
I(aq) +OCl(aq) → IO¯¯(aq) + Cl¯(aq)
Experiment
[I-] M
0.005
[OCI-]
0.005
Initial Rate M/min
0.000275
0.0025
0.005
0.000138
0.0025
0.0025
Calculate the overall order of this reaction using the table data.
0.0025
0.000069
0.0025
0.000140
Chapter 18 Solutions
EBK ORGANIC CHEMISTRY
Ch. 18.1 - Draw the structure for each of the following: a....Ch. 18.3 - Why does hydration inactivate FeBr3?Ch. 18.6 - Prob. 4PCh. 18.7 - What is the major product of a Friedel-Crafts...Ch. 18.9 - Describe two ways to prepare each of the following...Ch. 18.10 - Prob. 7PCh. 18.11 - Name the following:Ch. 18.11 - Draw a structure for each of the following: a....Ch. 18.11 - Draw the structure for each of the following: a....Ch. 18.11 - Correct the following incorrect names: a....
Ch. 18.12 - Prob. 14PCh. 18.12 - List the compounds in each set from most reactive...Ch. 18.13 - Prob. 16PCh. 18.13 - What product(s) result from nitration of each of...Ch. 18.13 - Prob. 18PCh. 18.13 - What products are obtained from the reaction of...Ch. 18.15 - Give the products, if any, of each of the...Ch. 18.16 - a. Does a coupling reaction have to be used to...Ch. 18.16 - Show how the following compounds can be...Ch. 18.16 - Prob. 24PCh. 18.17 - What is the major product(s) of each of the...Ch. 18.17 - Prob. 26PCh. 18.18 - Why isn't FeBr3 used as a catalyst in the first...Ch. 18.18 - Prob. 29PCh. 18.18 - Write the sequence of steps required for the...Ch. 18.18 - Show how the following compounds can be...Ch. 18.19 - What product is formed from reaction of...Ch. 18.19 - Prob. 33PCh. 18.19 - Draw the structure of the activated ring and the...Ch. 18.20 - Prob. 35PCh. 18.20 - Prob. 36PCh. 18.20 - Diazomethane can be used to convert a carboxylic...Ch. 18.21 - Prob. 38PCh. 18.21 - Prob. 39PCh. 18.21 - Prob. 40PCh. 18.22 - Prob. 41PCh. 18 - Draw the structure for each of the following: a....Ch. 18 - Name the following:Ch. 18 - Prob. 44PCh. 18 - Prob. 45PCh. 18 - For each of the statements in Column I, choose a...Ch. 18 - What product is obtained from the reaction of...Ch. 18 - Draw the product(s) of each of the following...Ch. 18 - Rank the following substituted anilines from most...Ch. 18 - Prob. 50PCh. 18 - Prob. 51PCh. 18 - Show how the following compounds can be...Ch. 18 - Prob. 53PCh. 18 - The compound with the 1H NMR spectrum shown below...Ch. 18 - Rank each group of compounds from most reactive to...Ch. 18 - Prob. 56PCh. 18 - Prob. 57PCh. 18 - For each of the following components, indicate the...Ch. 18 - Prob. 59PCh. 18 - Prob. 60PCh. 18 - Describe two ways to prepare anisole from benzene.Ch. 18 - Prob. 62PCh. 18 - The following tertiary alkyl bromides undergo an...Ch. 18 - An aromatic hydrocarbon with a molecular formula...Ch. 18 - Show how the following compounds can be...Ch. 18 - Use the four compounds shown below to answer the...Ch. 18 - a. Rank the following esters from most reactive to...Ch. 18 - A mixture of 0.10 mol benzene and 0.10 mol...Ch. 18 - Prob. 69PCh. 18 - Prob. 70PCh. 18 - Benzene underwent a Friedel-Crafts acylation...Ch. 18 - Prob. 72PCh. 18 - Prob. 73PCh. 18 - Friedel-Crafts alkylations can be carried out with...Ch. 18 - Show how the following compounds can be prepared...Ch. 18 - Prob. 76PCh. 18 - Prob. 77PCh. 18 - a. Describe four ways the following reaction can...Ch. 18 - Propose a mechanism for each of the following...Ch. 18 - How can you prepare the following compounds with...Ch. 18 - Describe how naphthalene can he prepared from the...Ch. 18 - Using resonance contributors for the carbocation...Ch. 18 - Prob. 83PCh. 18 - What reagents are required to carry out the...Ch. 18 - Prob. 85PCh. 18 - Prob. 86PCh. 18 - Prob. 87PCh. 18 - Propose a mechanism for each of the following...Ch. 18 - P-Fluoronitrobenzene is more reactive toward...Ch. 18 - When heated with chromic acid, compound A forms...Ch. 18 - Show how the following compounds can be prepared...Ch. 18 - How can you distinguish the following compounds...Ch. 18 - Describe how mescaline can be synthesized from...Ch. 18 - Propose a mechanism for the following reaction...Ch. 18 - Propose a mechanism for each of the following...Ch. 18 - Describe how 3-methyl-1-phenyl-3-pentanol can he...Ch. 18 - An unknown compound reacts with ethyl chloride and...Ch. 18 - a. Explain why the following reaction leads to the...Ch. 18 - Explain why hydroxide ion catalyzes the reaction...Ch. 18 - Prob. 100PCh. 18 - Prob. 101PCh. 18 - a. How can aspirin be synthesized from benzene? b....Ch. 18 - Prob. 103PCh. 18 - Show how Novocain, a painkiller used frequently by...Ch. 18 - Prob. 105PCh. 18 - Saccharin, an artificial sweetener, is about 300...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- H2O2(aq) +3 I¯(aq) +2 H+(aq) → 13(aq) +2 H₂O(l)· ••• Experiment [H2 O2]o (M) [I]o (M) [H+]。 (M) Initial rate (M/s) 1 0.15 0.15 0.05 0.00012 234 0.15 0.3 0.05 0.00024 0.3 0.15 0.05 0.00024 0.15 0.15 0.1 0.00048 Calculate the overall order of this reaction using the table data.arrow_forwardThe U. S. Environmental Protection Agency (EPA) sets limits on healthful levels of air pollutants. The maximum level that the EPA considers safe for lead air pollution is 1.5 μg/m³ Part A If your lungs were filled with air containing this level of lead, how many lead atoms would be in your lungs? (Assume a total lung volume of 5.40 L.) ΜΕ ΑΣΦ = 2.35 1013 ? atoms ! Check your rounding. Your final answer should be rounded to 2 significant figures in the last step. No credit lost. Try again.arrow_forwardY= - 0.039 (14.01) + 0.7949arrow_forward
- Suppose 1.76 g of magnesium acetate (Mg (CH3CO2)2) are dissolved in 140. mL of water. Find the composition of the resulting electrolyte solution. In particular, list the chemical symbols (including any charge) of each dissolved ion in the table below. List only one ion per row. mEq Then, calculate the concentration of each ion in dwrite the concentration in the second column of each row. Be sure you round your answers to the L correct number of significant digits. ion Add Row mEq L x 5arrow_forwardA pdf file of your hand drawn, stepwise mechanisms for the reactions. For each reaction in the assignment, you must write each mechanism three times (there are 10 reactions, so 30 mechanisms). (A) do the work on a tablet and save as a pdf., it is expected to write each mechanism out and NOT copy and paste the mechanism after writing it just once. Everything should be drawn out stepwise and every bond that is formed and broken in the process of the reaction, and is expected to see all relevant lone pair electrons and curved arrows.arrow_forwardNonearrow_forward
- Nonearrow_forwardDraw the structure of the product of the reaction given the IR and MS data. Spectral analysis of the product reveals: MS: M 150, M-15, M-43 CH.COCI AICI, IR: 3150-3000 cm, 2950-2850 cm and 1700 cmarrow_forwardPart II. Identify whether the two protons in blue are homotopic, enantiopic, diasteriotopic, or heterotopic. a) HO b) Bri H HH c) d) H H H Br 0arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
IR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=_TmevMf-Zgs;License: Standard YouTube License, CC-BY