
(a)
Pressure and volume at given points.
(a)
Answer to Problem 61P
Volume at A, B, C, D are
Pressure at B and D are
Explanation of Solution
Write the ideal gas equation and rearrange for
Here
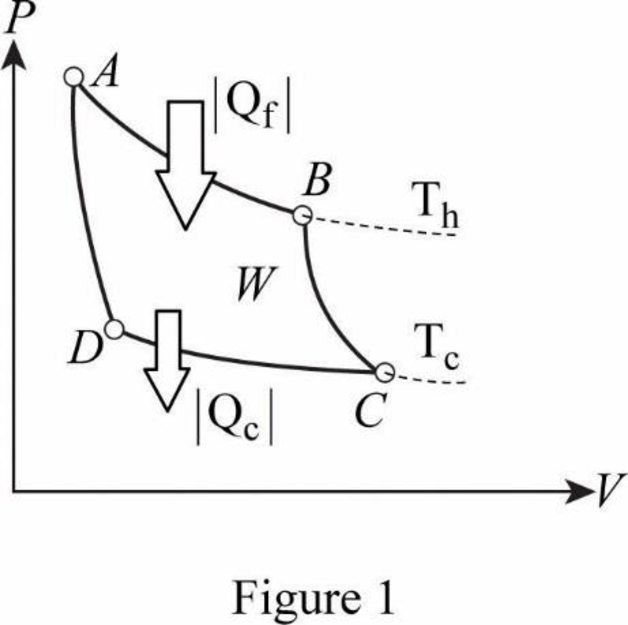
From figure, AB is isothermal.
Therefore.
Here
BC is adiabatic.
Therefore,
Here
Rearrange (II) in terms of
Rearrange (IV) in terms of
Similarly,
CD is isothermal.
Here
AD is adiabatic.
Substitute for
Rearrange (VIII) in terms of
Rearrange (II) and (VI) in terms of
Conclusion:
Substitute
Substitute
Substitute
Substitute
Volume at A, B, C, D are
Pressure at B and D are
(b)
Net work done per cycle.
(b)
Answer to Problem 61P
Net work done is
Explanation of Solution
Write the first law of
Here
As the change in internal energy is zero, (XI) becomes,
Write the equation for work done
Here
Write the equation for efficiency
Here
Rewrite (XIIII) in terms of
Conclusion:
Substitute
Net work done is
Want to see more full solutions like this?
Chapter 18 Solutions
Bundle: Principles of Physics: A Calculus-Based Text, 5th + WebAssign Printed Access Card for Serway/Jewett's Principles of Physics: A Calculus-Based Text, 5th Edition, Multi-Term
- 3.19 • Win the Prize. In a carnival booth, you can win a stuffed gi- raffe if you toss a quarter into a small dish. The dish is on a shelf above the point where the quarter leaves your hand and is a horizontal dis- tance of 2.1 m from this point (Fig. E3.19). If you toss the coin with a velocity of 6.4 m/s at an angle of 60° above the horizontal, the coin will land in the dish. Ignore air resistance. (a) What is the height of the shelf above the point where the quarter leaves your hand? (b) What is the vertical component of the velocity of the quarter just before it lands in the dish? Figure E3.19 6.4 m/s 2.1arrow_forwardCan someone help me answer this thank you.arrow_forward1.21 A postal employee drives a delivery truck along the route shown in Fig. E1.21. Determine the magnitude and direction of the resultant displacement by drawing a scale diagram. (See also Exercise 1.28 for a different approach.) Figure E1.21 START 2.6 km 4.0 km 3.1 km STOParrow_forward
- help because i am so lost and it should look something like the picturearrow_forward3.31 A Ferris wheel with radius Figure E3.31 14.0 m is turning about a horizontal axis through its center (Fig. E3.31). The linear speed of a passenger on the rim is constant and equal to 6.00 m/s. What are the magnitude and direction of the passenger's acceleration as she passes through (a) the lowest point in her circular motion and (b) the high- est point in her circular motion? (c) How much time does it take the Ferris wheel to make one revolution?arrow_forward1.56 ⚫. Three horizontal ropes pull on a large stone stuck in the ground, producing the vector forces A, B, and C shown in Fig. P1.56. Find the magnitude and direction of a fourth force on the stone that will make the vector sum of the four forces zero. Figure P1.56 B(80.0 N) 30.0 A (100.0 N) 53.0° C (40.0 N) 30.0°arrow_forward
- 1.39 Given two vectors A = -2.00 +3.00 +4.00 and B=3.00 +1.00 -3.00k. (a) find the magnitude of each vector; (b) use unit vectors to write an expression for the vector difference A - B; and (c) find the magnitude of the vector difference A - B. Is this the same as the magnitude of B - Ä? Explain.arrow_forward5. The radius of a circle is 5.5 cm. (a) What is the circumference in meters? (b) What is its area in square meters? 6. Using the generic triangle below, solve the following: 0 = 55 and c = 32 m, solve for a and b. a = 250 m and b = 180 m, solve for the angle and c. b=104 cm and c = 65 cm, solve for a and the angle b a 7. Consider the figure below representing the Temperature (T in degrees Celsius) as a function of time t (in seconds) 4 12 20 (a) What is the area under the curve in the figure below? (b) The area under the graph can be calculated using integrals or derivatives? (c) During what interval is the derivative of temperature with respect to time equal to zero?arrow_forwardPart 3: Symbolic Algebra Often problems in science and engineering are done with variables only. Don't let the different letters confuse you. Manipulate them algebraically as though they were numbers. 1. Solve 3x-7= x + 3 for x 2x-1 2. Solve- for x 2+2 In questions 3-11 solve for the required symbol/letter 3. v2 +2a(s-80), a = = 4. B= Ho I 2π r 5. K = kz² 6.xm= MAL ,d= d 7.T, 2 = 8.F=Gm 9. mgh=mv² 10.qV = mu² 80 12. Suppose that the height in meters of a thrown ball after t seconds is given by h =6+4t-t². Complete the square to find the highest point and the time when this happens. 13. Solve by completing the square c₁t² + cat + 3 = 0. 14. Solve for the time t in the following expression = 0 + vot+at²arrow_forward
- A blacksmith cools a 1.60 kg chunk of iron, initially at a temperature of 650.0° C, by trickling 30.0°C water over it. All the water boils away, and the iron ends up at a temperature of 120.0° C. For related problem-solving tips and strategies, you may want to view a Video Tutor Solution of Changes in both temperature and phase. Part A How much water did the blacksmith trickle over the iron? Express your answer with the appropriate units. HÅ mwater = Value 0 ? Units Submit Request Answerarrow_forwardSteel train rails are laid in 13.0-m-long segments placed end to end. The rails are laid on a winter day when their temperature is -6.0° C. Part A How much space must be left between adjacent rails if they are just to touch on a summer day when their temperature is 32.0°C? Express your answer with the appropriate units. ☐ о μΑ ? D = Value Units Submit Previous Answers Request Answer × Incorrect; Try Again; 3 attempts remaining Al Study Tools Looking for some guidance? Let's work through a few related practice questions before you go back to the real thing. This won't impact your score, so stop at anytime and ask for clarification whenever you need it. Ready to give it a try? Start Part B If the rails are originally laid in contact, what is the stress in them on a summer day when their temperature is 32.0°C? Express your answer in pascals. Enter positive value if the stress is tensile and negative value if the stress is compressive. F A Ο ΑΣΦ ? Раarrow_forwardhelp me with this and the step I am so confused. It should look something like the figure i shownarrow_forward
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning





