
Fundamentals of Physics
10th Edition
ISBN: 9781118230718
Author: David Halliday
Publisher: Wiley, John & Sons, Incorporated
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 18, Problem 21P
SSM ILW As a result of a temperature rise of 32 C°, a bar with a crack at its center buckles upward (Fig. 18-32). The fixed distance
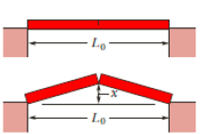
Figure 18-32 Problem 21.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
No chatgpt pls will upvote
Asap
A satellite has a mass of 100kg and is located at 2.00 x 10^6 m above the surface of the earth. a) What is the potential energy associated with the satellite at this loction? b) What is the magnitude of the gravitational force on the satellite?
No chatgpt pls will upvote
Chapter 18 Solutions
Fundamentals of Physics
Ch. 18 - The initial length L, change in temperature T, and...Ch. 18 - Figure 18-24 shows three linear temperature...Ch. 18 - Materials A, B, and C are solids that are at their...Ch. 18 - A sample A of liquid water and a sample B of ice,...Ch. 18 - Question 4 continued: Graphs b through f of Fig....Ch. 18 - Figure 18-26 shows three different arrangements of...Ch. 18 - Figure 18-27 shows two closed cycles on p-V...Ch. 18 - For which cycle in Fig. 18-27, traversed...Ch. 18 - Three different materials of identical mass are...Ch. 18 - A solid cube of edge length r, a solid sphere of...
Ch. 18 - A hot object is dropped into a thermally insulated...Ch. 18 - Suppose the temperature of a gas is 373.15 K when...Ch. 18 - Two constant-volume gas thermometers are...Ch. 18 - A gas thermometer is constructed of two...Ch. 18 - a In 1964, the temperature in the Siberian village...Ch. 18 - At what temperature is the Fahrenheit scale...Ch. 18 - On a linear X temperature scale, water freezes at...Ch. 18 - ILW Suppose that on a linear temperature scale X,...Ch. 18 - At 20C, a brass cube has edge length 30 cm. What...Ch. 18 - ILW A circular hole in an aluminum plate is 2.725...Ch. 18 - An aluminum flagpole is 33 m high. By how much...Ch. 18 - Prob. 11PCh. 18 - An aluminum-alloy rod has a length of 10.000 cm at...Ch. 18 - SSM Find the change in volume of an aluminum...Ch. 18 - When the temperature of a copper coin is raised by...Ch. 18 - ILW A steel rod is 3.000 cm in diameter at 25.00C....Ch. 18 - When the temperature of a metal cylinder is raised...Ch. 18 - SSM WWW An aluminum cup of 100 cm3 capacity is...Ch. 18 - At 20C, a rod is exactly 20.05 cm long on a steel...Ch. 18 - GO A vertical glass tube of length L = 1.280 000 m...Ch. 18 - GO In a certain experiment, a small radioactive...Ch. 18 - SSM ILW As a result of a temperature rise of 32 C,...Ch. 18 - One way to keep the contents of a garage from...Ch. 18 - SSM A small electric immersion healer is used to...Ch. 18 - A certain substance has a mass per mole of 50.0...Ch. 18 - Prob. 25PCh. 18 - What muss of butter, which has a usable energy...Ch. 18 - SSM Calculate the minimum amount of energy, in...Ch. 18 - How much water remains unfrozen after 50.2 kJ is...Ch. 18 - In a solar water heater, energy from the Sun is...Ch. 18 - A 0.400 kg simple is placed in a cooling apparatus...Ch. 18 - ILW What mass of steam at 100C must be mixed with...Ch. 18 - The specific heat of a substance varies with...Ch. 18 - Nonmetric version: a How long does a 2.0 105...Ch. 18 - GO Samples A and B are at different initial...Ch. 18 - An insulated Thermos contains l30 cm3 of hot...Ch. 18 - A 150 g copper bowl contains 220 g of water, both...Ch. 18 - A person makes a quantity of iced tea by mixing...Ch. 18 - A 0.530 kg sample of liquid water and a sample of...Ch. 18 - GO Ethyl alcohol has a boiling point of 78.0C, a...Ch. 18 - GO Calculate the specific heat of a metal from the...Ch. 18 - SSM WWW a Two 50 g ice cubes are dropped into 200...Ch. 18 - GO A 20.0 g copper ring at 0.000C has an inner...Ch. 18 - In Fig. 18-37, a gas sample expands from V0 to...Ch. 18 - GO A thermodynamic system is taken from stale A to...Ch. 18 - SSM ILW A gas within a closed chamber undergoes...Ch. 18 - Suppose 200 J of work is done on a system and 70.0...Ch. 18 - Prob. 47PCh. 18 - GO As a gas is held within a closed chamber, it...Ch. 18 - GO Figure 18-42 represents a closed cycle for a...Ch. 18 - GO A lab sample of gas is taken through cycle abca...Ch. 18 - A sphere of radius 0.500 m, temperature 27.0C, and...Ch. 18 - The ceiling of a single-family dwelling in a cold...Ch. 18 - SSM Consider the slab shown in Fig. 18-18. Suppose...Ch. 18 - If you were to walk briefly in space without a...Ch. 18 - ILW A cylindrical copper rod of length 1.2 m and...Ch. 18 - The giant hornet Vespa mandarinia japonica preys...Ch. 18 - Prob. 57PCh. 18 - A solid cylinder of radius r1 = 2.5 cm, length h1...Ch. 18 - Prob. 59PCh. 18 - GO Figure 18-46 shows the cross section of a wall...Ch. 18 - SSM A 5.0 cm slap has formed on an outdoor tank of...Ch. 18 - Leidenfrost effect. A water drop will last about 1...Ch. 18 - GO Figure 18-49 shows in cross section a wall...Ch. 18 - Prob. 64PCh. 18 - Ice has formed on a shallow pond, and a shady...Ch. 18 - GO Evaporative cooling of beverages. A cold...Ch. 18 - In the extrusion of cold chocolate from a tube,...Ch. 18 - Prob. 68PCh. 18 - Figure 18-51 displays a closed cycle for a gas....Ch. 18 - In a certain solar house, energy from the Sun is...Ch. 18 - A 0.300 kg sample is placed in a cooling apparatus...Ch. 18 - The average rate at which energy is conducted...Ch. 18 - What is the volume increase of an aluminum cube...Ch. 18 - In a series of experiment, block B is to be placed...Ch. 18 - Figure 18-54 displays a dosed cycle for a gas....Ch. 18 - Three equal-length straight rods, of aluminum,...Ch. 18 - SSM The temperature of a 0.700 kg cube of ice is...Ch. 18 - GO Icicles. Liquid water coats an active growing...Ch. 18 - SSM A sample of gas expands from an initial...Ch. 18 - Figure 18-56a shows a cylinder containing gas and...Ch. 18 - SSM A sample of gas undergoes a transition from an...Ch. 18 - Prob. 82PCh. 18 - SSM The temperature of a Pyrex disk is changed...Ch. 18 - a Calculate the rate at which body heat is...Ch. 18 - SSM A 2.50 kg Jump of aluminum is heated to 92.0C...Ch. 18 - A glass window pane is exactly 20 cm by 30 cm at...Ch. 18 - A recruit can join the semi-secret 300 F club at...Ch. 18 - A steel rod at 25.0C is bolted at both ends and...Ch. 18 - An athlete needs to lose weight and decides to do...Ch. 18 - Soon after Earth was formed, heat released by the...Ch. 18 - Prob. 91PCh. 18 - A rectangular plate of glass initially has the...Ch. 18 - Suppose that you intercept 5.0 103 of the energy...Ch. 18 - A thermometer of mass 0.0550 kg and of specific...Ch. 18 - A sample of gas expands from V1 = 1.0 m3 and p1 =...Ch. 18 - Figure 18-59 shows a composite bar of length L =...Ch. 18 - On finding your stove out of order, you decide to...Ch. 18 - The p-V diagram in the Fig. 18-60 shows two paths...Ch. 18 - A cube of edge length 6.0 106 m, emissivity 0.75,...Ch. 18 - A flow calorimeter is a device used to measure the...Ch. 18 - An object of mass 6.00 kg falls through a height...Ch. 18 - The Pyrex glass mirror in a telescope has a...Ch. 18 - The area A of a rectangular plate is ab = 1.4 m2....Ch. 18 - Consider the liquid in a barometer whose...Ch. 18 - A pendulum clock with a pendulum made of brass is...Ch. 18 - Prob. 106PCh. 18 - Prob. 107PCh. 18 - A 1700 kg Buick moving at 83 km/h brakes to a...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Describe an example of bioconversion. What metabolic processes can result in fuels?
Microbiology: An Introduction
7. Two rubber bands pulling on an object cause it to accelerate at 1.2 m/s2.
a. What will be the object’s acc...
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Two parents plan to have three children. What is the probability that the children will be two girls and one bo...
Genetic Analysis: An Integrated Approach (3rd Edition)
45. Calculate the mass of nitrogen dissolved at room temperature in an 80.0-L home aquarium. Assume a total pre...
Chemistry: Structure and Properties (2nd Edition)
4. Two of these organ system bear the major responsibility for ensuring homeostasis of the internal environment...
Human Anatomy & Physiology (Marieb, Human Anatomy & Physiology) Standalone Book
Heat lamps are commonly used to maintain foods at about 50C for as long as 12 hours in cafeteria serving lines....
Microbiology: An Introduction
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Correct answer No chatgpt pls will upvotearrow_forwardStatistical thermodynamics. The number of imaginary replicas of a system of N particlesa) cannot be greater than Avogadro's numberb) must always be greater than Avogadro's number.c) has no relation to Avogadro's number.arrow_forwardLab-Based Section Use the following information to answer the lab based scenario. A student performed an experiment in an attempt to determine the index of refraction of glass. The student used a laser and a protractor to measure a variety of angles of incidence and refraction through a semi-circular glass prism. The design of the experiment and the student's results are shown below. Angle of Incidence (°) Angle of Refraction (º) 20 11 30 19 40 26 50 31 60 36 70 38 2a) By hand (i.e., without using computer software), create a linear graph on graph paper using the student's data. Note: You will have to manipulate the data in order to achieve a linear function. 2b) Graphically determine the index of refraction of the semi-circular glass prism, rounding your answer to the nearest hundredth.arrow_forward
- Use the following information to answer the next two questions. A laser is directed at a prism made of zircon (n = 1.92) at an incident angle of 35.0°, as shown in the diagram. 3a) Determine the critical angle of zircon. 35.0° 70° 55 55° 3b) Determine the angle of refraction when the laser beam leaves the prism.arrow_forwardUse the following information to answer the next two questions. A laser is directed at a prism made of zircon (n = 1.92) at an incident angle of 35.0°, as shown in the diagram. 3a) Determine the critical angle of zircon. 35.0° 70° 55 55° 3b) Determine the angle of refraction when the laser beam leaves the prism.arrow_forwardNo chatgpt pls will upvotearrow_forward
- A beam of alpha-particles of energy 7.3MeV is used.The protons emitted at an angle of zero degree are found to have energy of 9.34MeV.Find the Q-value of this reaction .arrow_forwardAn aluminum rod and a copper rod have the same length of 100cm at 5C. At what temperatures would one of the rods be 0.5 mm longer than the other? Which rod is longer at such temperature?arrow_forwardROTATIONAL DYNAMICS Question 01 A solid circular cylinder and a solid spherical ball of the same mass and radius are rolling together down the same inclined. Calculate the ratio of their kinetic energy. Assume pure rolling motion Question 02 A sphere and cylinder of the same mass and radius start from ret at the same point and more down the same plane inclined at 30° to the horizontal Which body gets the bottom first and what is its acceleration b) What angle of inclination of the plane is needed to give the slower body the same acceleration Question 03 i) Define the angular velocity of a rotating body and give its SI unit A car wheel has its angular velocity changing from 2rads to 30 rads seconds. If the radius of the wheel is 400mm. calculate ii) The angular acceleration iii) The tangential linear acceleration of a point on the rim of the wheel Question 04 in 20arrow_forward
- Question B3 Consider the following FLRW spacetime: t2 ds² = -dt² + (dx² + dy²+ dz²), t2 where t is a constant. a) State whether this universe is spatially open, closed or flat. [2 marks] b) Determine the Hubble factor H(t), and represent it in a (roughly drawn) plot as a function of time t, starting at t = 0. [3 marks] c) Taking galaxy A to be located at (x, y, z) = (0,0,0), determine the proper distance to galaxy B located at (x, y, z) = (L, 0, 0). Determine the recessional velocity of galaxy B with respect to galaxy A. d) The Friedmann equations are 2 k 8πG а 4πG + a² (p+3p). 3 a 3 [5 marks] Use these equations to determine the energy density p(t) and the pressure p(t) for the FLRW spacetime specified at the top of the page. [5 marks] e) Given the result of question B3.d, state whether the FLRW universe in question is (i) radiation-dominated, (ii) matter-dominated, (iii) cosmological-constant-dominated, or (iv) none of the previous. Justify your answer. f) [5 marks] A conformally…arrow_forwardSECTION B Answer ONLY TWO questions in Section B [Expect to use one single-sided A4 page for each Section-B sub question.] Question B1 Consider the line element where w is a constant. ds²=-dt²+e2wt dx², a) Determine the components of the metric and of the inverse metric. [2 marks] b) Determine the Christoffel symbols. [See the Appendix of this document.] [10 marks] c) Write down the geodesic equations. [5 marks] d) Show that e2wt it is a constant of geodesic motion. [4 marks] e) Solve the geodesic equations for null geodesics. [4 marks]arrow_forwardPage 2 SECTION A Answer ALL questions in Section A [Expect to use one single-sided A4 page for each Section-A sub question.] Question A1 SPA6308 (2024) Consider Minkowski spacetime in Cartesian coordinates th = (t, x, y, z), such that ds² = dt² + dx² + dy² + dz². (a) Consider the vector with components V" = (1,-1,0,0). Determine V and V. V. (b) Consider now the coordinate system x' (u, v, y, z) such that u =t-x, v=t+x. [2 marks] Write down the line element, the metric, the Christoffel symbols and the Riemann curvature tensor in the new coordinates. [See the Appendix of this document.] [5 marks] (c) Determine V", that is, write the object in question A1.a in the coordinate system x'. Verify explicitly that V. V is invariant under the coordinate transformation. Question A2 [5 marks] Suppose that A, is a covector field, and consider the object Fv=AAμ. (a) Show explicitly that F is a tensor, that is, show that it transforms appropriately under a coordinate transformation. [5 marks] (b)…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
 Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning University Physics Volume 1PhysicsISBN:9781938168277Author:William Moebs, Samuel J. Ling, Jeff SannyPublisher:OpenStax - Rice University
University Physics Volume 1PhysicsISBN:9781938168277Author:William Moebs, Samuel J. Ling, Jeff SannyPublisher:OpenStax - Rice University College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning


Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

University Physics Volume 1
Physics
ISBN:9781938168277
Author:William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:OpenStax - Rice University

College Physics
Physics
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning
Heat Transfer: Crash Course Engineering #14; Author: CrashCourse;https://www.youtube.com/watch?v=YK7G6l_K6sA;License: Standard YouTube License, CC-BY