
Essential University Physics: Volume 1 (3rd Edition)
3rd Edition
ISBN: 9780321993724
Author: Richard Wolfson
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 18, Problem 21E
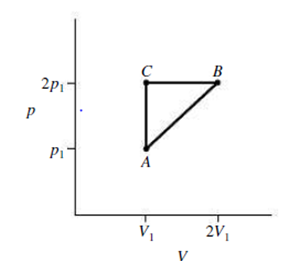
Repeat Exercise 20 for a process that follows the path ACB in Fig. 18.19.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
A bat is flying toward a cave wall at 27.0 m/s. What is the frequency of the reflected sound that it hears, assuming it emits sound at 52.0 kHz? The speed of sound
is 341.5 m/s.
Multiple Choice
о
60.9 kHz
О
56.5 kHz
о
61.3 kHz
О
56.1 kHz
Compare the slope of your Data Table 2 graph to the average wavelength (Ave, l) from Data Table 2 by calculating the % Difference. Is the % Difference calculated for the wavelength in Data Table 2 within an acceptable % error? Explain why or why not?
The slope of a graph of velocity, v, vs frequency, f, is equal to wavelength, l. Compare the slope of your Data Table 1 graph to the average wavelength (Ave, l) from Data Table 1 by calculating the % Difference.
Chapter 18 Solutions
Essential University Physics: Volume 1 (3rd Edition)
Ch. 18.2 - Two identical gas-cylinder systems are taken from...Ch. 18.2 - Name the basic thermodynamic process involved when...Ch. 18.3 - The same amount of heat flows into equal volumes...Ch. 18 - Prob. 1FTDCh. 18 - Prob. 2FTDCh. 18 - Prob. 3FTDCh. 18 - Why cant an irreversible process be described by a...Ch. 18 - Are the initial and final equilibrium states of an...Ch. 18 - Does the first law of thermodynamics apply to...Ch. 18 - Prob. 7FTD
Ch. 18 - Figure 18.18 shows two processes, A and B. that...Ch. 18 - When you let air out of a tire, the air seems...Ch. 18 - Blow on the back of your hand with your mouth wide...Ch. 18 - You boil water in an open pan. Of which of the...Ch. 18 - Three identical gas-cylinder systems are...Ch. 18 - Prob. 13FTDCh. 18 - In what sense can a gas of diatomic molecules be...Ch. 18 - Prob. 15ECh. 18 - Prob. 16ECh. 18 - A 40-W heat source is applied to a gas sample for...Ch. 18 - Find the rate of heat flow into a system whose...Ch. 18 - In a certain automobile engine, 17% of the total...Ch. 18 - An ideal gas expands from the state (p1, V1) to...Ch. 18 - Repeat Exercise 20 for a process that follows the...Ch. 18 - A balloon contains 0.30 mol of helium. It rises,...Ch. 18 - The balloon of Exercise 22 starts at 100 kPa...Ch. 18 - How much work does it take to compress 2.5 mol of...Ch. 18 - By what factor must the volume of a gas with =...Ch. 18 - Prob. 26ECh. 18 - A carbon-sequestration scheme calls for...Ch. 18 - A gas mixture contains 2.5 mol of O2 and 3.0 mol...Ch. 18 - A mixture of monatomic and diatomic gases has...Ch. 18 - What should be the approximate specific-heat ratio...Ch. 18 - Prob. 31ECh. 18 - An ideal gas expands to 10 times its original...Ch. 18 - During cycling, the human body typically releases...Ch. 18 - A 0.25-mol sample of ideal gas initially occupies...Ch. 18 - As the heart beats, blood pressure in an artery...Ch. 18 - It takes 1.5 kJ to compress a gas isothermally to...Ch. 18 - A gas undergoes an adiabatic compression during...Ch. 18 - A gas with = 1.40 occupies 6.25 L when its at...Ch. 18 - A gas sample undergoes the cyclic process ABCA...Ch. 18 - Prob. 40PCh. 18 - A gasoline engine has compression ratio 8.5 (sec...Ch. 18 - By what factor must the volume of a gas with =...Ch. 18 - Volvos B5340 engine, used in the V70 series cars,...Ch. 18 - A research balloon is prepared for launch by...Ch. 18 - Prob. 45PCh. 18 - By what factor does the internal energy of an...Ch. 18 - An ideal monatomic gas is compressed to half its...Ch. 18 - A gas expands isothermally from state A to state...Ch. 18 - A 3.50-mol sample of ideal gas with molar specific...Ch. 18 - Prove that the slope of an adiabat at a given...Ch. 18 - An ideal gas with = 1.67 starts at point A in...Ch. 18 - The gas of Example 18.4 starts at state A in Fig....Ch. 18 - The gas of Example 18.4 starts at state A in Fig....Ch. 18 - A 25-L sample of ideal gas with = 1.67 is at 250...Ch. 18 - Prob. 55PCh. 18 - A 25-L sample of ideal gas with = 1.67 is at 250...Ch. 18 - Youre the product safety officer for a company...Ch. 18 - Figure 18.22 shows data and a fit curve from an...Ch. 18 - External forces compress 21 mol of ideal monatomic...Ch. 18 - A gas with = 7/5 is at 273 K when its compressed...Ch. 18 - An ideal gas with = 1.3 is initially at 273 K and...Ch. 18 - The curved path in Fig. 18.23 lies on the 350-K...Ch. 18 - Repeat part (a) of Problem 62 for the path ACDA in...Ch. 18 - A gas mixture contains monatomic argon and...Ch. 18 - How much of a triatomic gas with Cv = 3R would you...Ch. 18 - An 8.5-kg rock at 0C is dropped into a...Ch. 18 - A piston-cylinder arrangement containing 0.30 mol...Ch. 18 - Experimental studies show that the pV curve for a...Ch. 18 - Show that the application of Equation 18.3 to an...Ch. 18 - A horizontal piston-cylinder system containing n...Ch. 18 - Prob. 71PCh. 18 - The table below shows measured values of pressure...Ch. 18 - In a reversible process, a volume of air V0= 17 m3...Ch. 18 - A real gas is more accurately described using the...Ch. 18 - Repeat Exercise 20 for an expansion along the path...Ch. 18 - The adiabatic lapse rate is the rate at which air...Ch. 18 - The nuclear power plant at which youre the public...Ch. 18 - Prob. 78PCh. 18 - One scheme for reducing greenhouse-gas emissions...Ch. 18 - Warm winds called Chinooks (a Native-American term...Ch. 18 - Warm winds called Chinooks (a Native-American term...Ch. 18 - Warm winds called Chinooks (a Native-American term...Ch. 18 - Warm winds called Chinooks (a Native-American term...
Additional Science Textbook Solutions
Find more solutions based on key concepts
33. Consider the reaction:
The tabulated data were collected for the concentration of C4H8 as a function...
Chemistry: Structure and Properties (2nd Edition)
A variety of pea plant called Blue Persian produces a tall plant with blue seeds. A second variety of pea plant...
Genetic Analysis: An Integrated Approach (3rd Edition)
1.1 Write a one-sentence definition for each of the following:
a. chemistry
b. chemical
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Fibrous connective tissue consists of ground substance and fibers that provide strength, support, and flexibili...
Human Biology: Concepts and Current Issues (8th Edition)
60. People immersed in strong unchanging magnetic fields occasionally report sensing a metallic taste. Some inv...
College Physics: A Strategic Approach (3rd Edition)
Distinguish between microevolution, speciation, and macroevolution.
Campbell Essential Biology (7th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Examine the slope of the line on the graph created using the data in Data Table 4 of Period, T2 vs L, the slope of the line is a constant containing the acceleration due to gravity, g. Using the slope of your line, determine the experimental value for g. Compare the value you determined for g from the slope of the graph to the expected value of 9.81 m/s2 by calculating the percent error.arrow_forwardFrom your examination of the graph created using the data in Data Table 4 of Period, T vs √L . What would you determine is the relationship between the period of a pendulum and the length of a pendulum?arrow_forwardIn a certain bimetallic strip, the brass strip is 0.100% longer than the steel strip at a temperature of 283°C. At what temperature do the two strips have the same length? Coefficients of linear expansion for steel α = 12.0 × 10−6 K−1 and for brass α = 19.0 × 10−6 K−1 (see Table 13.2).arrow_forward
- Review Conceptual Example 2 before attempting this problem. Two slits are 0.158 mm apart. A mixture of red light (wavelength = 693 nm) and yellow-green light (wavelength = 567 nm) falls on the slits. A flat observation screen is located 2.42 m away. What is the distance on the screen between the third-order red fringe and the third-order yellow-green fringe? m = 3 m = 3 m = 0 m = 3 m = 3 Fringes on observation screenarrow_forwardA film of oil lies on wet pavement. The refractive index of the oil exceeds that of the water. The film has the minimum nonzero thickness such that it appears dark due to destructive interference when viewed in visible light with wavelength 643 nm in vacuum. Assuming that the visible spectrum extends from 380 to 750 nm, what is the longest visible wavelength (in vacuum) for which the film will appear bright due to constructive interference? Number Unitsarrow_forwardA piece of metal is placed on top of a 2.0 - kg wooden block (mass density = 562 kg/m³) piece. UseArchimedes' principle to calculate the mass (in kg) of copper if the top of the wood surface is exactly at thewater's surface?arrow_forward
- A filmmaker wants to achieve an interesting visual effect by filming a scene through a converging lens with a focal length of 50.0 m. The lens is placed betwen the camera and a horse, which canters toward the camera at a constant speed of 7.9 m/s. The camera starts rolling when the horse is 36.0 m from the lens. Find the average speed of the image of the horse (a) during the first 2.0 s after the camera starts rolling and (b) during the following 2.0 s.arrow_forwardAnswer the question (Physics)arrow_forwardsolve smybolically and plug in numbers and solve at the endarrow_forward
- answer the question symbolically until you have to plug in numbers. show all work please.arrow_forwardWhat is the direction of the magnetic force on a NEGATIVE CHARGE that moves as shown in each of the six cases?arrow_forwardHi! I need help with these calculations for part i and part k for a physics Diffraction Lab. We used a slit width 0.4 mm to measure our pattern.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning
The Second Law of Thermodynamics: Heat Flow, Entropy, and Microstates; Author: Professor Dave Explains;https://www.youtube.com/watch?v=MrwW4w2nAMc;License: Standard YouTube License, CC-BY