
Concept explainers
(a)
Interpretation:
The structural formula for the principal product formed when benzamide is treated with the given reagent has to be drawn.
Concept introduction:
The normal reaction of an amide with water does not give good yield of product. As amides are least reactive
The reaction of water and amides in presence of acid gives a carboxylic acid as a product. The reaction equation is written as,
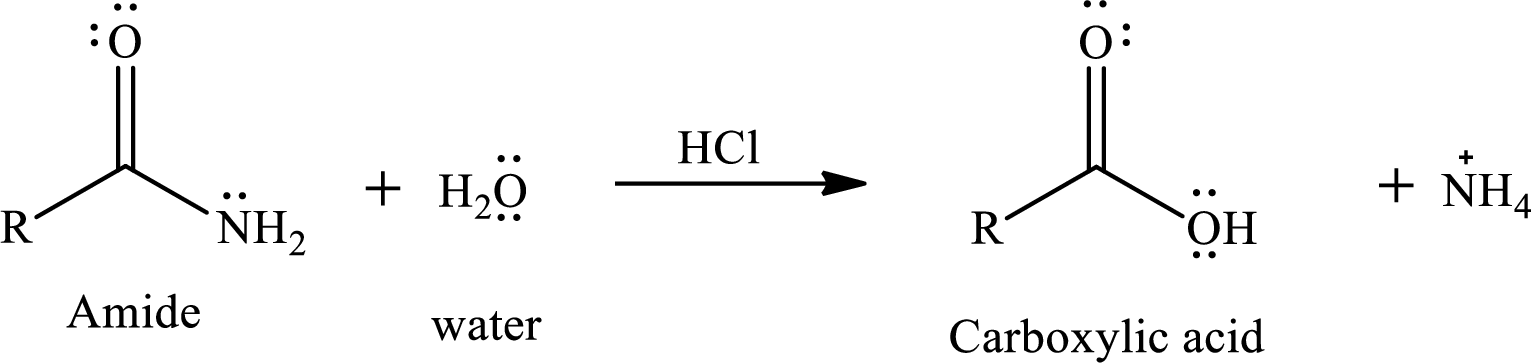
The reactivity of amide also depends upon the leaving tendency of amine part from acyl group. The weaker the base better will be the leaving group. The basicity of
(b)
Interpretation:
The structural formula for the principal product formed when benzamide is treated with the given reagent has to be drawn.
Concept introduction:
Amide hydrolysis in aqueous base:
A carboxylate salt and ammonia or an amine will be the product of amide hydrolysis in aqueous base.
For each mole of amide, one mole of base is required.
(c)
Interpretation:
The structural formula for the principal product formed when benzamide is treated with the given reagent has to be drawn.
Concept introduction:
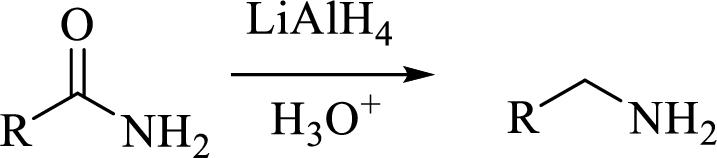
Trending nowThis is a popular solution!

Chapter 18 Solutions
OWLv2 with MindTap Reader, 1 term (6 months) Printed Access Card for Brown/Iverson/Anslyn/Foote's Organic Chemistry, 8th Edition
- State the products (formulas) of the reaction of acetophenone with iodine and NaOH.arrow_forwardExplanation Check Draw the skeletal ("line") structure of 5-hydroxy-4-methyl-2-pentanone. Click and drag to start drawing a structure. Х © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Cer ☐ : Carrow_forward1. Using a Model set Build a model for the following compound [CH2BrCI]. 2. Build another model of the mirror image of your first molecule. 3. Place the two models next to each other and take a picture which shows the differences between the two models. 4. Determine the absolute stereochemistry R or S for the two models. 5. Write or type a paragraph to Discuss the stereochemical relationship between the two models of CH2BrCl. You must provide an explanation for your conclusions also provide a description for the colors used to represent each atom in the model's images.arrow_forward
- What parameters are included in the specific rotation calculation of a pure substance based on measurement from a polarimeter? Select one or more: Density of the sample Pathlength of the sample container Enantiomeric excess of the sample Measured rotation of lightarrow_forwardV Determine whether the following molecule is a hemiacetal, acetal, or neither and select the appropriate box below. Also, highlight the hemiacetal or acetal carbon if there is one. Explanation O CH O Ohemiacetal Oacetal Oneither Check A 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Cer 000 Ararrow_forward1. Using Online resources and chemical structures hand draw four different organic compounds (not those already shown in your handout) that are chiral, optically active (a pair of enantiomers will count as one). Pay attention to correct stereochemistry 2. Write or type a short paragraph to Discuss the stereochemical relationship between the four compounds.arrow_forward
- 1. Using a Model set Build a model for the following compound [CHBRIF] 2. Build another model of the mirror image of your first molecule. 3. Place the two models next to each other and take a picture which shows the differences between the two models. 4. Determine the absolute stereochemistry R or S for the two models. 5. Write or type a paragraph to Discuss the stereochemical relationship between the two models of CHBгCIF. You must provide an explanation for your conclusions also provide a description for the colors used to representarrow_forwardThe specific rotation of a sample depends upon measured angle of rotation, the density of the sample, and the pathway length of the light. True Falsearrow_forwardConsider the molecule A,B, C and D shown below, (1 x 4) Br NH2 A OH Br 边 H B C D 1. Assign the R/S configuration to each chiral center and identify by circling all the chiral centers. 2. Draw an image for the enantiomer of each of the compounds A, B, C and D.arrow_forward
- Could you crystallize one enantiomer of mandelic acid from a racemic mixture (using the typical achiral solvents found in our lab) without preparing a diastereomeric salt? Why or why not? No, because both enantiomers have the same solubility in achiral solvents. than the other. ооо Yes, because one enantiomer has a higher melting point No, because both enantiomers are liquids. Yes, because one enantiomer is more crystalline than the other.arrow_forwardIf the literature value of specific rotation for a chiral compound is -53.6°, what is the enantiomeric excess of a compound with a measured specific rotation of -40.5°?arrow_forwardThe process to determine the configuration, starts by placing the lowest priority substituent toward the back. If the substituents pointing forward decrease in priority in a clockwise order, the configuration is S. If the substituents decrease in priority in a counterclockwise order, the configuration is R. True Falsearrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

