
Concept explainers
(a)
Interpretation:
The structural formula for the principal product formed when benzonitrile is treated with the given reagent has to be drawn.
Concept introduction:
The reaction of
The hydrolysis reaction of alkyl nitrile in presence of acid is written as,
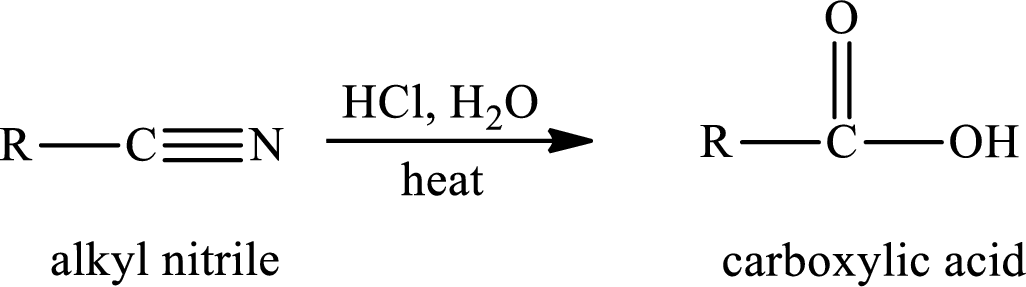
The amide is first formed when the cyano group is hydrolysed in excess of water and if there is only one mole of water per mole of nitrile, the reaction can be stopped by the formation of amide.
(b)
The structural formula for the principal product formed when benzonitrile is treated with the given reagent has to be drawn.
Concept introduction:
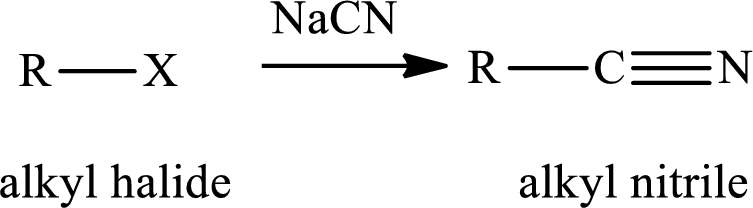
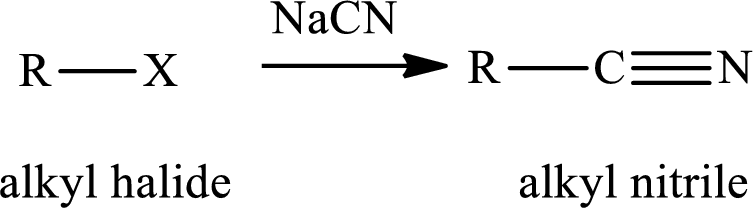
The reaction of alkyl halides with sodium cyanide gives alkyl cyanides or nitriles. After the reaction there is increase incarbon atoms of the alkyl chain of alkyl halide. The nitriles obtained undergo hydrolysis reaction in presence of acid gives carboxylic acid. The general reaction of alkyl halides and sodum cyanide is written as,
The hydrolysis reaction of alkyl nitrile in presence of acid is written as,
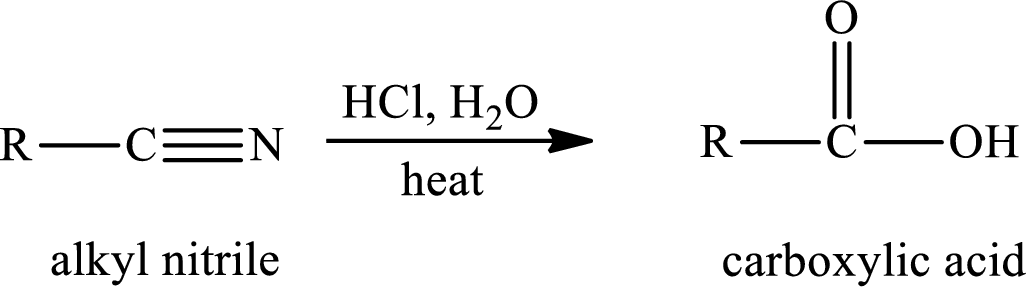
The amide is first formed when the cyano group is hydrolysed in excess of water and if there is only one mole of water per mole of nitrile, the reaction can be stopped by the formation of amide.
(c)
The structural formula for the principal product formed when benzonitrile is treated with the given reagent has to be drawn.
Concept introduction:
Amide hydrolysis in aqueous base:
A carboxylate anion and ammonia or an
The formed carboxylate anion can be converted into carboxylic acid by the acidification of the reaction mixture.
(d)
The structural formula for the principal product formed when benzonitrile is treated with the given reagent has to be drawn.
Concept introduction:
Concept introduction:
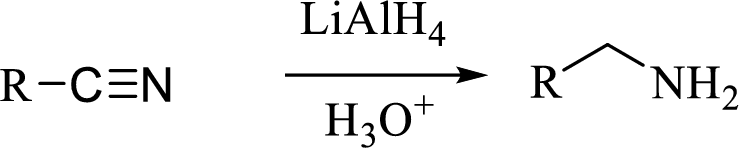
Trending nowThis is a popular solution!

Chapter 18 Solutions
OWLv2 with MindTap Reader, 1 term (6 months) Printed Access Card for Brown/Iverson/Anslyn/Foote's Organic Chemistry, 8th Edition
- Steps and explanations pleasearrow_forwardUse diagram to answer the following: 1.Is the overall rxn endo- or exothermic. Explain briefly your answer____________________2. How many steps in this mechanism?_____________3. Which is the rate determining step? Explain briefly your answer____________________4. Identify (circle and label) the reactants,the products and intermediate (Is a Cation, Anion, or a Radical?) Please explain and provide full understanding.arrow_forwardDraw the entire mechanism and add Curved Arrows to show clearly how electrons areredistributed in the process. Please explain and provide steps clearly.arrow_forward
- Match the denticity to the ligand. Water monodentate ✓ C₂O2 bidentate H₂NCH₂NHCH2NH2 bidentate x EDTA hexadentate Question 12 Partially correct Mark 2 out of 2 Flag question Provide the required information for the coordination compound shown below: Na NC-Ag-CN] Number of ligands: 20 Coordination number: 2✔ Geometry: linear Oxidation state of transition metal ion: +3 x in 12 correct out of 2 question Provide the required information for the coordination compound shown below. Na NC-Ag-CN] Number of ligands: 20 Coordination number: 2 Geometry: linear 0 Oxidation state of transition metal ion: +3Xarrow_forwardCan you explain step by step behind what the synthetic strategy would be?arrow_forwardPlease explain step by step in detail the reasoning behind this problem/approach/and answer. thank you!arrow_forward
- 2. Predict the product(s) that forms and explain why it forms. Assume that any necessary catalytic acid is present. .OH HO H₂N OHarrow_forwardconsider the rate of the reaction below to be r. Whats the rate after each reaction? Br + NaCN CN + NaBr a. Double the concentration of alkyl bromide b. Halve the concentration of the electrophile & triple concentration of cyanide c. Halve the concentration of alkyl chloridearrow_forwardPredict the organic reactant that is involved in the reaction below, and draw the skeletal ("line") structures of the missing organic reactant. Please include all steps & drawings & explanations.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning



