
Concept explainers
(a)
Interpretation:
The products formed when given compound is treated with
Concept introduction:
Benzene undergoes electrophile substitution. The kinetics of the electrophilic substitution reaction depends upon the nature of substituent present on the benzene ring. Electron releasing groups activates the ring towards the electrophilic substitution reaction while electron withdrawing groups deactivates the ring towards the electrophilic substitution reaction.
Answer to Problem 18.15P
The product formed by the reaction of given compound with
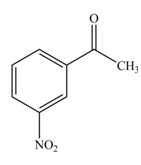
The reaction occurs slower because benzene ring contains deactivating group.
Explanation of Solution
Electron withdrawing groups deactivates the ring towards the electrophilic substitution reaction.
The substituent present in the given compound is electron withdrawing group. Thus, it directs the electrophile to meta position and deactivates the ring towards the electrophilic substitution reaction. Hence, the given compound reacts slower than benzene. The reaction is shown below.
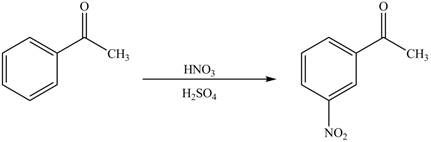
Figure 1
The product formed by the reaction of given compound with
(b)
Interpretation:
The products formed when given compound is treated with
Concept introduction:
Benzene undergoes electrophile substitution. The kinetics of the electrophilic substitution reaction depends upon the nature of substituent present on the benzene ring. Electron releasing groups activates the ring towards the electrophilic substitution reaction while electron withdrawing groups deactivates the ring towards the electrophilic substitution reaction.
Answer to Problem 18.15P
The product formed by the reaction of given compound with
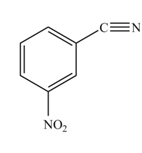
The reaction occurs slower because benzene ring contains deactivating group.
Explanation of Solution
Electron withdrawing groups deactivates the ring towards the electrophilic substitution reaction.
The substituent present in the given compound is electron withdrawing group. Thus it directs the electrophile to meta position and deactivates the ring towards the electrophilic substitution reaction. Hence, the given compound reacts slower than benzene. The reaction is shown below.
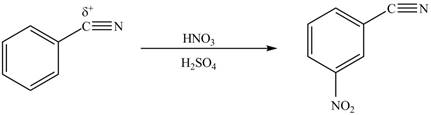
Figure 2
The product formed by the reaction of given compound with
(c)
Interpretation:
The products formed when given compound is treated with
Concept introduction:
Benzene undergoes electrophile substitution. The kinetics of the electrophile substitution reaction depends upon the nature of substituent present on the benzene ring. Electron releasing groups activates the ring towards the electrophilic substitution reaction while electron withdrawing groups deactivates the ring towards the electrophilic substitution reaction.
Answer to Problem 18.15P
The product formed by the reaction of given compound with
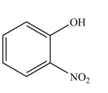
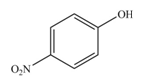
The reaction occurs faster because benzene ring contains activating group.
Explanation of Solution
Electron releasing group directs the electrophile to ortho and para position.
The substituent present in the given compound is electron donating group. Thus, it directs the electrophile to ortho and para position and activates the ring towards the electrophilic substitution reaction. Hence, the given compound reacts faster than benzene. The reaction is shown below.
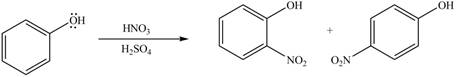
Figure 3
The product formed by the reaction of given compound with
(d)
Interpretation:
The products formed when given compound is treated with
Concept introduction:
Benzene undergoes electrophile substitution. The kinetics of the electrophilic substitution reaction depends upon the nature of substituent present on the benzene ring. Electron releasing groups activates the ring towards the electrophilic substitution reaction while electron withdrawing groups deactivates the ring towards the electrophilic substitution reaction.
Answer to Problem 18.15P
The products formed by the reaction of given compound with
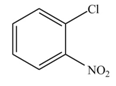
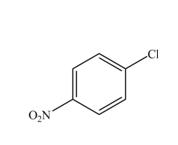
The reaction occurs slower benzene ring because benzene ring contains deactivating group.
Explanation of Solution
The substituent present in the given compound is
Among these two cases, mesomeric effect predominates over inductive effect. Hence, chlorine on benzene ring acts as releasing group but deactivates the benzene ring due to its
Thus, it directs the electrophile to ortho and para position and deactivates the ring towards the electrophilic substitution reaction. Hence, the given compound reacts slower than benzene. The reaction is shown below.
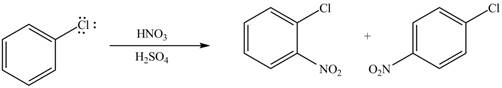
Figure 4
The product formed by the reaction of given compound with
(e)
Interpretation:
The products formed when given compound is treated with
Concept introduction:
Benzene undergoes electrophile substitution. The kinetics of the electrophile substitution reaction depends upon the nature of substituent present on the benzene ring. Electron releasing groups activates the ring towards the electrophilic substitution reaction while electron withdrawing groups deactivates the ring towards the electrophilic substitution reaction.
Answer to Problem 18.15P
The products formed by the reaction of given compound with
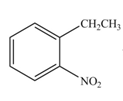
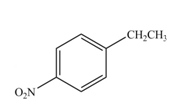
The reaction occurs higher because benzene ring contains activating group.
Explanation of Solution
Electron releasing group directs the electrophile to ortho and para positions.
The substituent present in the given compound is electron withdrawing group. Thus it directs the electrophile to ortho and para positions and activates the ring towards the electrophilic substitution reaction. Hence, the given compound reacts faster than benzene. The reaction is shown below.
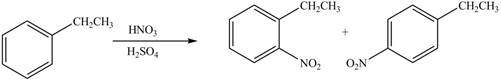
Figure 5
The product formed by the reaction of given compound with
Want to see more full solutions like this?
Chapter 18 Solutions
Organic Chemistry
- b) Certain cyclic compounds are known to be conformationally similar to carbohydrates, although they are not themselves carbohydrates. One example is Compound C shown below, which could be imagined as adopting four possible conformations. In reality, however, only one of these is particularly stable. Circle the conformation you expect to be the most stable, and provide an explanation to justify your choice. For your explanation to be both convincing and correct, it must contain not only words, but also "cartoon" orbital drawings contrasting the four structures. Compound C Possible conformations (circle one): Детarrow_forwardLab Data The distance entered is out of the expected range. Check your calculations and conversion factors. Verify your distance. Will the gas cloud be closer to the cotton ball with HCI or NH3? Did you report your data to the correct number of significant figures? - X Experimental Set-up HCI-NH3 NH3-HCI Longer Tube Time elapsed (min) 5 (exact) 5 (exact) Distance between cotton balls (cm) 24.30 24.40 Distance to cloud (cm) 9.70 14.16 Distance traveled by HCI (cm) 9.70 9.80 Distance traveled by NH3 (cm) 14.60 14.50 Diffusion rate of HCI (cm/hr) 116 118 Diffusion rate of NH3 (cm/hr) 175.2 175.2 How to measure distance and calculate ratearrow_forwardFor the titration of a divalent metal ion (M2+) with EDTA, the stoichiometry of the reaction is typically: 1:1 (one mole of EDTA per mole of metal ion) 2:1 (two moles of EDTA per mole of metal ion) 1:2 (one mole of EDTA per two moles of metal ion) None of the abovearrow_forward
- Please help me solve this reaction.arrow_forwardIndicate the products obtained by mixing 2,2-dimethylpropanal with acetaldehyde and sodium ethoxide in ethanol.arrow_forwardSynthesize 2-Ethyl-3-methyloxirane from dimethyl(propyl)sulfonium iodide using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Synthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- If possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forwardSynthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIndicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

