
Concept explainers
Synthesize each compound from benzene and any other organic or inorganic reagents.
a.
b.
c. 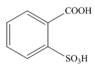 d.
d. 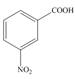 e.
e. ![]() f.
f. 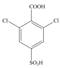 g.
g. 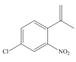
(a)
Interpretation: The synthesis of given compound from benzene and any other organic or inorganic reagent is to be stated.
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 18.64P
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown below:
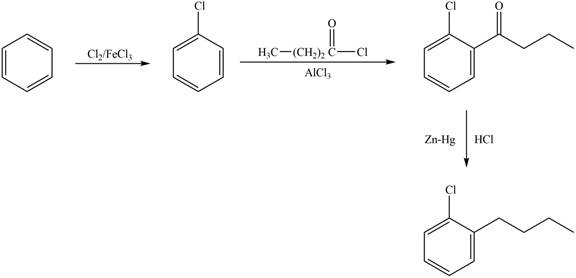
Explanation of Solution
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown in Figure 1.
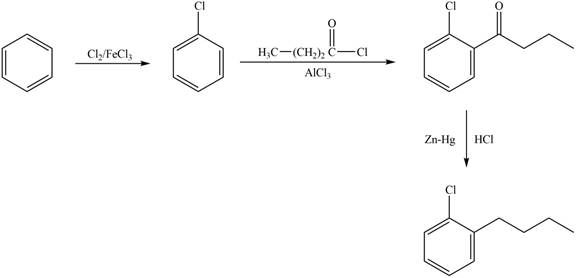
Figure 1
The first step in the synthesis is the chlorination of benzene. The next step is the Friedel-Craft acylation in which chlorobenzene reacts with butnoyl chloride. The last step is the clemmensen reduction to form the desired product.
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown in Figure 1.
(b)
Interpretation: The synthesis of given compound from benzene and any other organic or inorganic reagent is to be stated.
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 18.64P
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown below:
Explanation of Solution
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown in Figure 2.

Figure 2
The first step in the synthesis is the formation of nitrobenzene. The next step is the reaction of nitrobenzene with bromine in the presence of aluminum chloride to give the final product.
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown in Figure 2.
(c)
Interpretation: The synthesis of given compound from benzene and any other organic or inorganic reagent is to be stated.
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 18.64P
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown below.
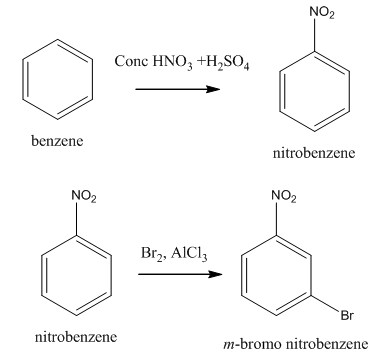
Explanation of Solution
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown in Figure 3.

Figure 3
The first step in the synthesis is the conversion of benzene into toluene. The next step is the reaction of toluene with concentrated sulfuric acid which results the addition of sulphonyl group at ortho position. The last step is the oxidation of methyl group into acid in the presence of potassium permanganate to give the final product.
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown in Figure 3.
(d)
Interpretation: The synthesis of given compound from benzene and any other organic or inorganic reagent is to be stated.
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 18.64P
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown below:
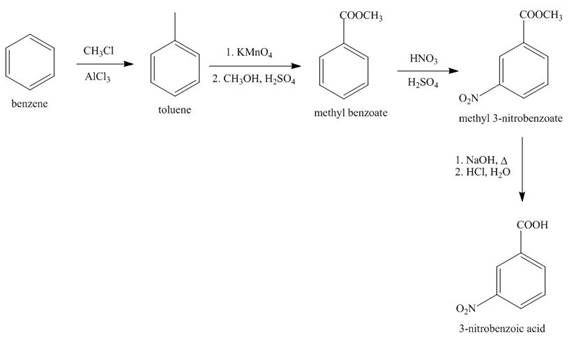
Explanation of Solution
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown in Figure 4.

Figure 4
The first step in the synthesis is the conversion of benzene into toluene. The next step is the oxidation of methyl group into acid in the presence of potassium permanganate to give benzoic acid followed by the reaction with methanol to form methyl benzoate. The reaction of nitric acid in the presence of sulphuric acid results in the formation of final product.
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown in Figure 4.
(e)
Interpretation: The synthesis of given compound from benzene and any other organic or inorganic reagent is to be stated.
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 18.64P
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown below:
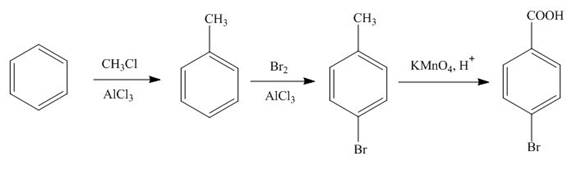
Explanation of Solution
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown in Figure 5.
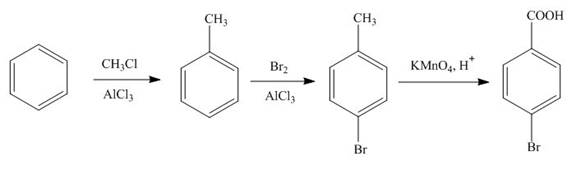
Figure 5
The first step in the synthesis is the conversion of benzene into toluene. The next step is the reaction of toluene with bromine in the presence of aluminum chloride. The last step is the oxidation of methyl group into acid in the presence of potassium permanganate to give the final product.
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown in Figure 5.
(f)
Interpretation: The synthesis of given compound from benzene and any other organic or inorganic reagent is to be stated.
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 18.64P
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown below:
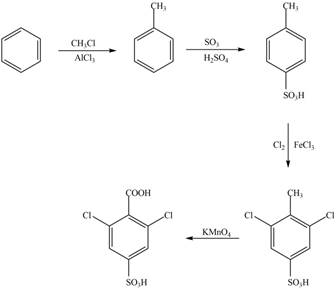
Explanation of Solution
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown in Figure 6.

Figure 6
The synthesis of given compound take place in four steps: Friedel-Craft alkylation, sulfonation, chlorination and at last oxidation.
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown in Figure 6.
(g)
Interpretation: The synthesis of given compound from benzene and any other organic or inorganic reagent is to be stated.
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron deficient chemical species that contains positive charge are known as electrophile. In electrophilic aromatic substitution reaction, electrophile takes the position of hydrogen atom by attacking the electron rich carbon atom of benzene.
Answer to Problem 18.64P
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown below:
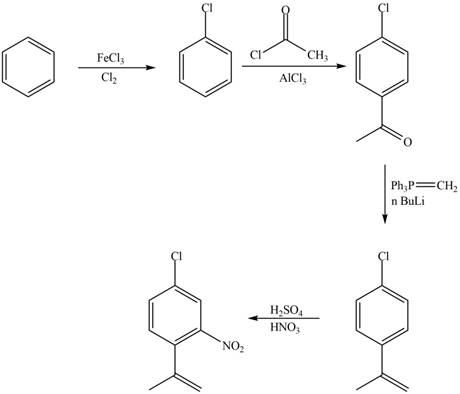
Explanation of Solution
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown in Figure 3.
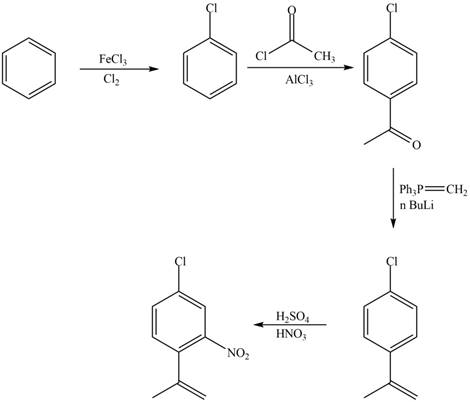
Figure 7
The first, second, third and fourth step involved in the synthesis of given compound is chlorination, Friedel-Craft acylation, Wittig reaction and nitration, respectively.
The synthesis of given compound from benzene and any other organic or inorganic reagent is shown in Figure 7.
Want to see more full solutions like this?
Chapter 18 Solutions
Organic Chemistry
- QUESTION 1 Write the IUPAC names for the following compounds. (a) (b) 2 H₂C CH (c) Br (d) HO (e) COOHarrow_forwardneed help finding the product of these reactionsarrow_forwardPart 1. Draw monomer units of the following products and draw their reaction mechanism 1) Bakelite like polymer Using: Resorcinol + NaOH + Formalin 2) Polyester fiber Using a) pthalic anhydride + anhydrous sodium acetate + ethylene glycol B)pthalic anhydride + anhydrous sodium acetate + glycerol 3) Temporary cross-linked polymer Using: 4% polyvinyl alcohol+ methyl red + 4% sodium boratearrow_forward
- Using the table of Reactants and Products provided provide the correct letter that corresponds with the Carboxylic acid that is formed in the reaction below. 6 M NaOH Acid-workup WRITE THE CORRECT LETTER ONLY DO NOT WRITE EXTRA WORDS OR PHRASES A) Pool of Reagents for Part B CI B) OH C) E) CI J) racemic F) K) OH N) OH P) G) OH D) HO H) L) M) HO Q) R) CI Aarrow_forwardIn the table below, the exact chemical structures for Methyl salicylate can be represented by the letter WRITE THE CORRECT LETTER ONLY DO NOT WRITE EXTRA WORDS OR PHRASES CI B) A) E) Cl racemic F) J) CI K) N) OH P) Pool of Reagents for Part B OH OH G) L) OH D) HO H) M) HO Q) R) CIarrow_forwardDraw the stepwise mechanism for the reactionsarrow_forward
- Part I. a) Draw reaction mechanism for the transformations of benzophenone to benzopinacol to benzopinaco lone b) Pinacol (2,3-dimethyl, 1-3-butanediol) on treatment w/ acid gives a mixture of pina colone (3,3-dimethyl-2-butanone) and 2, 3-dimethyl - 1,3-butadiene. Give reasonable mechanism the formation of the products Forarrow_forward3. The explosive decomposition of 2 mole of TNT (2,4,6-trinitrotoluene) is shown below: Assume the C(s) is soot-basically atomic carbon (although it isn't actually atomic carbon in real life). 2 CH3 H NO2 NO2 3N2 (g)+7CO (g) + 5H₂O (g) + 7C (s) H a. Use bond dissociation energies to calculate how much AU is for this reaction in kJ/mol.arrow_forwardPart I. Draw reaction mechanism for the transformations of benzophenone to benzopinacol to benzopinaco lone and answer the ff: Pinacol (2,3-dimethyl, 1-3-butanediol) on treatment w/ acid gives a mixture of pina colone and (3,3-dimethyl-2-butanone) 2,3-dimethyl-1,3-butadiene. Give reasonable mechanism the formation of the products Forarrow_forward
- Show the mechanism for these reactionsarrow_forwardDraw the stepwise mechanismarrow_forwardDraw a structural formula of the principal product formed when benzonitrile is treated with each reagent. (a) H₂O (one equivalent), H₂SO₄, heat (b) H₂O (excess), H₂SO₄, heat (c) NaOH, H₂O, heat (d) LiAlH4, then H₂Oarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





