
CHEMISTRY+CHEM...HYBRID ED.(LL)>CUSTOM<
9th Edition
ISBN: 9781305020788
Author: John C.Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: CENGAGE C
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 18, Problem 14PS
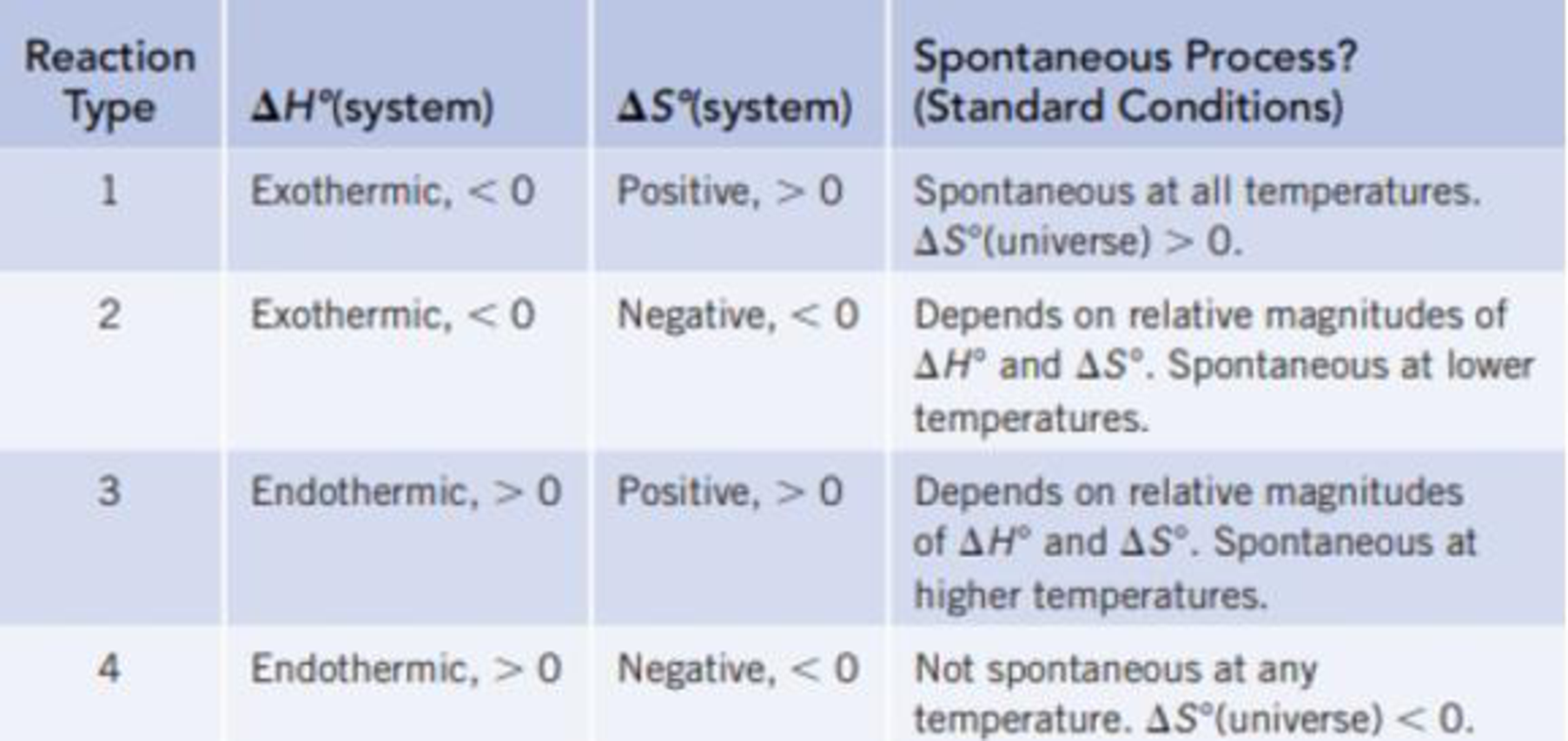
Classify each of the reactions according to one of the four reaction types summarized in Table 18.1.
- (a) C6H12O6(s) + 6 O2(g) → 6 CO2(g) + 6 H2O(ℓ)
ΔrH° = −673 kj/mol-rxn
ΔrS° = 60.4 j/K · mol-rxn
- (b) MgO(s) + C(graphite) → Mg(s) + CO(g)
ΔrH° = 490.7 kJ/mol-rxn
ΔrS° = 197.9 J/K · mol-rxn
TABLE 18.1 Predicting Whether a Reaction Will Be Spontaneous Under Standard Conditions
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
In the phase diagram of steel (two components Fe and C), region A is the gamma austenite solid and region B contains the gamma solid and liquid. Indicate the degrees of freedom that the fields A and B have,
For a condensed binary system in equilibrium at constant pressure, indicate the maximum number of phases that can exist.
Part V. Label ad match the carbons in compounds Jane and Diane
w/ the corresponding peak no.
in the
Spectra (Note: use the given peak no. To label the carbons, other peak
no are intentionally
omitted)
7 4 2
-0.13
-0.12
-0.11
-0.10
-0.08
8
CI
Jane
1
-0.09
5
210
200
190
180
170
160
150
140
130
120
110
100
-8
90
f1 (ppm)
11
8
172.4
172.0
f1 (ppr
HO
CI
NH
Diane
7
3
11
80
80
-80
-R
70
60
60
2
5
-8
50
40
8.
170
160
150
140
130
120
110
100
90
-0
80
70
20
f1 (ppm)
15
30
-20
20
-60
60
-0.07
-0.06
-0.05
-0.04
-0.03
-0.02
-0.01
-0.00
-0.01
10
-0.17
16
15
56
16
-0.16
-0.15
-0.14
-0.13
-0.12
-0.11
-0.10
-0.09
-0.08
-0.07
-0.06
-0.05
-0.04
17.8 17.6 17.4 17.2 17.0
f1 (ppm)
-0.03
-0.02
550
106
40
30
20
20
-0.01
-0.00
F-0.01
10
0
Chapter 18 Solutions
CHEMISTRY+CHEM...HYBRID ED.(LL)>CUSTOM<
Ch. 18.1 - 1. A process is spontaneous in the direction that...Ch. 18.1 - A process that is reactant-favored at equilibrium...Ch. 18.1 - Prob. 3RCCh. 18.2 - In a spontaneous process, S(universe) is (a) 0 (b)...Ch. 18.2 - 2. Which of the following is true for a...Ch. 18.2 - Prob. 3RCCh. 18.3 - Prob. 1RCCh. 18.3 - Prob. 2RCCh. 18.4 - Predict which substance in each pair has the...Ch. 18.4 - Prob. 2CYU
Ch. 18.4 - Without looking up their standard entropies in...Ch. 18.4 - Without doing any calculations, predict the sign...Ch. 18.4 - Calculate rS for the following reaction at 25 C....Ch. 18.5 - Based on rH and rS, predict the spontaneity of the...Ch. 18.5 - Prob. 1RCCh. 18.5 - Prob. 2RCCh. 18.5 - Prob. 3RCCh. 18.6 - Prob. 1RCCh. 18.6 - Prob. 2RCCh. 18.7 - Prob. 1CYUCh. 18.7 - Prob. 2CYUCh. 18.7 - Oxygen was first prepared by Joseph Priestley...Ch. 18.7 - Prob. 4CYUCh. 18.7 - Prob. 5CYUCh. 18.7 - Prob. 6CYUCh. 18.7 - Prob. 1RCCh. 18.7 - Prob. 2RCCh. 18.7 - Prob. 3RCCh. 18.7 - Consider the hydrolysis reactions of creatine...Ch. 18.7 - Prob. 2QCh. 18.A - The decomposition of diamond to graphite...Ch. 18.A - It has been demonstrated that buckminsterfullerene...Ch. 18 - Which substance has the higher entropy? (a) dry...Ch. 18 - Which substance has the higher entropy? (a) a...Ch. 18 - Use S values to calculate the standard entropy...Ch. 18 - Use S values to calculate the standard entropy...Ch. 18 - Calculate the standard entropy change for the...Ch. 18 - Calculate the standard entropy change for the...Ch. 18 - Calculate the standard entropy change for the...Ch. 18 - Calculate the standard entropy change for the...Ch. 18 - Is the reaction Si(s) + 2 Cl2(g) SiCl4(g)...Ch. 18 - Is the reaction Si(s) + 2 H2(g) SiH4(g)...Ch. 18 - Calculate S(universe) for the decomposition of 1...Ch. 18 - Calculate S(universe) for the formation of 1 mol...Ch. 18 - Classify each of the reactions according to one of...Ch. 18 - Classify each of the reactions according to one of...Ch. 18 - Using values of fH and S, calculate rG for each of...Ch. 18 - Using values of fH and S, calculate rG for each of...Ch. 18 - Using values of fH and S, calculate the standard...Ch. 18 - Using values of fH and S, calculate the standard...Ch. 18 - Using values of fG, calculate rG for each of the...Ch. 18 - Using values of fG, calculate rG for each of the...Ch. 18 - For the reaction BaCO3(s) BaO(s) + CO2(g), rG =...Ch. 18 - For the reaction TiCl2(s) + Cl2(g) TiCl4(), rG =...Ch. 18 - Determine whether the reactions listed below are...Ch. 18 - Determine whether the reactions listed below are...Ch. 18 - Heating some metal carbonates, among them...Ch. 18 - Calculate rH and rS for the reaction of tin(IV)...Ch. 18 - The standard free energy change, rG, for the...Ch. 18 - Prob. 28PSCh. 18 - Calculate rG at 25 C for the formation of 1.00 mol...Ch. 18 - Prob. 30PSCh. 18 - Prob. 31PSCh. 18 - Prob. 32PSCh. 18 - Compare the compounds in each set below and decide...Ch. 18 - Using standard entropy values, calculate rS for...Ch. 18 - About 5 billion kilograms of benzene, C6H6, are...Ch. 18 - Hydrogenation, the addition of hydrogen to an...Ch. 18 - Is the combustion of ethane, C2H6, product-favored...Ch. 18 - Prob. 38GQCh. 18 - When vapors from hydrochloric acid and aqueous...Ch. 18 - Calculate S(system), S(surroundings), and...Ch. 18 - Methanol is now widely used as a fuel in race...Ch. 18 - The enthalpy of vaporization of liquid diethyl...Ch. 18 - Calculate the entropy change, rS, for the...Ch. 18 - Using thermodynamic data, estimate the normal...Ch. 18 - Prob. 45GQCh. 18 - When calcium carbonate is heated strongly, CO2 gas...Ch. 18 - Sodium reacts violently with water according to...Ch. 18 - Yeast can produce ethanol by the fermentation of...Ch. 18 - Elemental boron, in the form of thin fibers, can...Ch. 18 - Prob. 50GQCh. 18 - Prob. 51GQCh. 18 - Estimate the boiling point of water in Denver,...Ch. 18 - The equilibrium constant for the butane ...Ch. 18 - A crucial reaction for the production of synthetic...Ch. 18 - Calculate rG for the decomposition of sulfur...Ch. 18 - Prob. 56GQCh. 18 - A cave in Mexico was recently discovered to have...Ch. 18 - Wet limestone is used to scrub SO2 gas from the...Ch. 18 - Sulfur undergoes a phase transition between 80 and...Ch. 18 - Calculate the entropy change for dissolving HCl...Ch. 18 - Some metal oxides can be decomposed to the metal...Ch. 18 - Prob. 62ILCh. 18 - Prob. 63ILCh. 18 - Prob. 64ILCh. 18 - Titanium(IV) oxide is converted to titanium...Ch. 18 - Cisplatin [cis-diamminedichloroplatinum(II)] is a...Ch. 18 - Prob. 67SCQCh. 18 - Explain why each of the following statements is...Ch. 18 - Decide whether each of the following statements is...Ch. 18 - Under what conditions is the entropy of a pure...Ch. 18 - Prob. 71SCQCh. 18 - Consider the formation of NO(g) from its elements....Ch. 18 - Prob. 73SCQCh. 18 - The normal melting point of benzene, C6H6, is 5.5...Ch. 18 - Prob. 75SCQCh. 18 - For each of the following processes, predict the...Ch. 18 - Heater Meals are food packages that contain their...Ch. 18 - Prob. 78SCQCh. 18 - Prob. 79SCQCh. 18 - Prob. 80SCQCh. 18 - Iodine, I2, dissolves readily in carbon...Ch. 18 - Prob. 82SCQCh. 18 - Prob. 83SCQCh. 18 - Prob. 84SCQCh. 18 - Prob. 85SCQCh. 18 - Prob. 86SCQCh. 18 - The Haber-Bosch process for the production of...Ch. 18 - Prob. 88SCQ
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- n Feb 3 A T + 4. (2 pts) Draw the structure of the major component of the Limonene isolated. Explain how you confirmed the structure. 5. (2 pts) Draw the fragment corresponding to the base peak in the Mass spectrum of Limonene. 6. (1 pts) Predict the 1H NMR spectral data of R-Limonene. Proton NMR: 5.3 pon multiplet (H Ringarrow_forwardPart VI. Ca H 10 O is the molecular formula of compound Tom and gives the in the table below. Give a possible structure for compound Tom. 13C Signals summarized C1 C2 C3 C4 C5 C6 C7 13C shift (ppm) 23.5 27.0 33.0 35.8 127 162 205 DEPT-90 + DEPT-135 + +arrow_forward2. Using the following data to calculate the value of AvapH o of water at 298K. AvapH o of water at 373K is 40.7 kJ/mol; molar heat capacity of liquid water at constant pressure is 75.2J mol-1 K-1 and molar heat capacity of water vapor at constant pressure is 33.6 J mol-1 K-1.arrow_forward
- Part VII. Below are the 'HNMR 13 3 C-NMR, COSY 2D- NMR, and HSQC 20-NMR (Similar with HETCOR but axes are reversed) spectra of an organic compound with molecular formula C6H13 O. Assign chemical shift values to the H and c atoms of the compound. Find the structure. Show complete solutions. Predicted 1H NMR Spectrum ли 4.7 4.6 4.5 4.4 4.3 4.2 4.1 4.0 3.9 3.8 3.7 3.6 3.5 3.4 3.3 3.2 3.1 3.0 2.9 2.8 2.7 2.6 2.5 2.4 2.3 2.2 2.1 2.0 1.9 1.8 1.7 1.6 1.5 1.4 1.3 1.2 1.1 1.0 0.9 0.8 f1 (ppm)arrow_forward3. Draw the expanded structural formula, the condensed structural formula, and the skeletal structural formula for 2-pentene. expanded structure: Condensed structure: Skeletal formula: 4. Draw the expanded structural formula, the condensed structural formula, and the skeletal structural formula for 2-methyl-3-heptene. expanded structure: Condensed structure: Skeletal formula: following structurearrow_forwardPart IV. Propose a plausible Structure w/ the following descriptions: a) A 5-carbon hydrocarbon w/ a single peak in its proton decoupled the DEPT-135 Spectrum shows a negative peak C-NMR spectrum where b) what cyclohexane dione isomer gives the largest no. Of 13C NMR signals? c) C5H120 (5-carbon alcohol) w/ most deshielded carbon absent in any of its DEPT Spectivaarrow_forward
- 13C NMR is good for: a) determining the molecular weight of the compound b) identifying certain functional groups. c) determining the carbon skeleton, for example methyl vs ethyl vs propyl groups d) determining how many different kinds of carbon are in the moleculearrow_forward6 D 2. (1 pt) Limonene can be isolated by performing steam distillation of orange peel. Could you have performed this experiment using hexane instead of water? Explain. 3. (2 pts) Using GCMS results, analyze and discuss the purity of the Limonene obtained from the steam distillation of orange peel.arrow_forwardPart III. Arrange the following carbons (in blue) in order of increasing chemical shift. HO B NH 2 A CIarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
Kinetics: Chemistry's Demolition Derby - Crash Course Chemistry #32; Author: Crash Course;https://www.youtube.com/watch?v=7qOFtL3VEBc;License: Standard YouTube License, CC-BY