
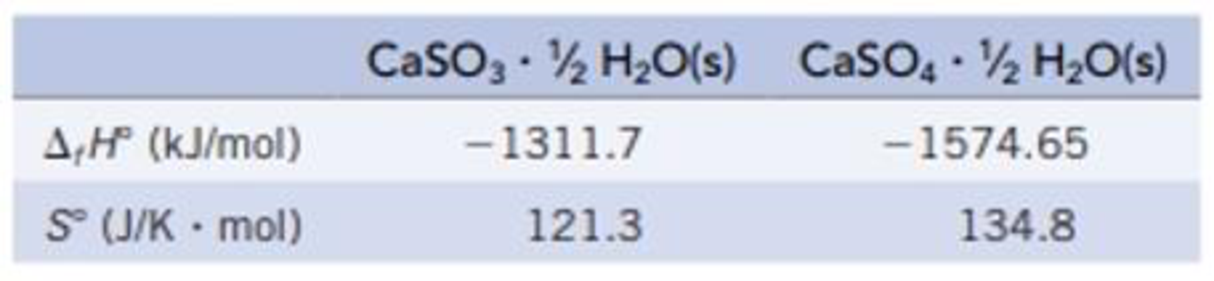
Wet limestone is used to scrub SO2 gas from the exhaust gases of power plants. One possible reaction gives hydrated calcium sulfite:
CaCO3(s) + SO2(g) + ½ H2O(ℓ) ⇄ CaSO3 · ½ H2O(s) + CO2(g)
Another reaction gives hydrated calcium sulfate:
CaCO3(s) + SO2(g) + ½ H2O(ℓ) + ½ O2(g) ⇄ CaSO4 · ½H2O(s) + CO2(g)
(a) Which reaction is more product-favored at equilibrium? Use the data in the table below and any other information needed in Appendix L to calculate ΔrG° for each reaction at 25 °C.
(b) Calculate ΔrG° for the reaction
CaSO3 · ½ H2O(s) + ½ O2(g) ⇄ CaSO4 · ½ H2O(s)
Is this reaction product- or reactant-favored at equilibrium?
(a)
Interpretation:
Among the given reactions the reactions that is more favoured at product side should be determined.
Concept introduction:
The Gibbs free energy or the free energy change is a thermodynamic quantity represented by
Here,
Answer to Problem 58GQ
The reaction which is more product-favored at equilibrium is reaction
Explanation of Solution
The value of
Given:
The Appendix L referred for the values of standard entropies and enthalpies.
For reaction
The standard enthalpy change is expressed as,
Substituting the respective values
Also,
Substituting the respective values
Now,
Substituting the value of
For reaction
The standard enthalpy change is expressed as,
Substituting the respective values
Also,
Substituting the respective values
Now,
Substituting the value of
(b)
Interpretation:
The
Concept introduction:
The Gibbs free energy or the free energy change is a thermodynamic quantity represented by
Here,
Answer to Problem 58GQ
The
Thus, the reaction is product-favored at equilibrium.
Explanation of Solution
The value of
Given:
The Appendix L referred for the values of standard entropies and enthalpies.
The standard enthalpy change is expressed as,
Substituting the respective values
Also,
Substituting the values,
Now,
Substitute the value of
Want to see more full solutions like this?
Chapter 18 Solutions
CHEMISTRY+CHEM...HYBRID ED.(LL)>CUSTOM<
- N Classify each of the following molecules as aromatic, antiaromatic, or nonaromatic. NH O aromatic O antiaromatic O nonaromatic O aromatic O antiaromatic O nonaromatic O aromatic O antiaromatic O nonaromatic Garrow_forwardThe conjugate base of alkanes is called alkides. Correct?.arrow_forwardName these organic compounds: structure Br name CH3 CH3 ☐ ☐arrow_forward
- HH H-C H -C-H HH Draw the Skeletal Structures & H Name the molecules HH H H H H-C-C-C-C-C-C-H HHH HHH H H HHHHHHH H-C-C-C-C-C-C-C-C-C-H HHHHH H H H Harrow_forwarddont provide AI solution .... otherwise i will give you dislikearrow_forwardName these organic compounds: structure name CH3 CH3 ☐ F F CH3 ☐ O Explanation Check 2025 McGraw Hill LLC. All Rights Reserved. Terms ofarrow_forward
- Classify each of the following molecules as aromatic, antiaromatic, or nonaromatic. ZI NH Explanation Check O aromatic O antiaromatic O nonaromatic O aromatic O antiaromatic H O nonaromatic O aromatic O antiaromatic O nonaromatic ×arrow_forwardPart I. Draw the stepwise reaction mechanism of each product (a, b, c, d, e, f) HO HO OH НОН,С HO OH Sucrose HO CH₂OH H N N HO -H H -OH KMnO4, Heat H OH CH₂OH (d) Phenyl Osatriazole OH НОН,С HO HO + Glacial HOAC HO- HO CH₂OH OH HO Fructose (a) Glucose OH (b) H₂N HN (c) CuSO4-5H2O, ethanol H N N N HO ·H H OH H OH N CH₂OH OH (f) Phenyl Osazone H (e) Carboxy phenyl osatriazole Figure 2.1. Reaction Scheme for the Total Synthesis of Fine Chemicalsarrow_forwardWhich molecule is the most stable? Please explain.arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning





