
Concept explainers
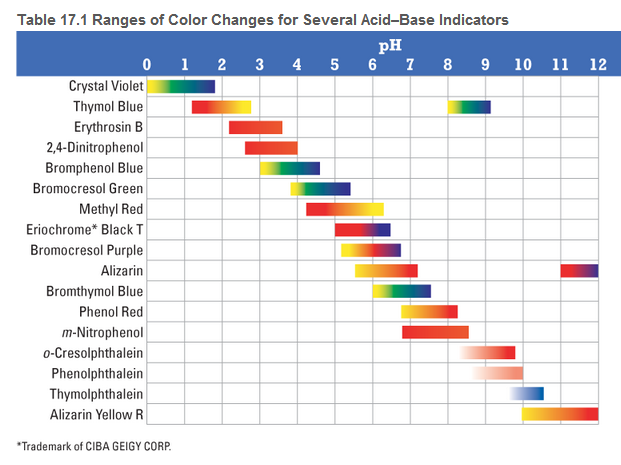
Sketch the titration curve from Problem 121 by calculating the pH at the beginning of the titration, at one-half of the equivalence point, at the equivalence point, and at 5.0 mL beyond the equivalence point. Pick a suitable indicator for this titration from Table 17.1
by calculating the pH at the beginning of the titration, at one-half of the equivalence point, at the equivalence point, and at 5.0 mL beyond the equivalence point. Pick a suitable indicator for this titration from Table 17.1 
121. A 0.552-g sample of ascorbic acid (vitamin C) is dissolved in water to a total volume of 20.0 mL and titrated with 0.1103 M KOH. The equivalence point occurs at 28.42 mL. The pH of the solution at 10.0 mL of added base was 3.72. From this data, determine the molar mass and Kafor vitamin C.
Want to see the full answer?
Check out a sample textbook solution
Chapter 18 Solutions
Chemistry: Structure and Properties Custom Edition for Rutgers University General Chemistry
Additional Science Textbook Solutions
Concepts of Genetics (12th Edition)
Organic Chemistry
MARINE BIOLOGY
Fundamentals of Anatomy & Physiology (11th Edition)
- Show work with explanation needed. Don't give Ai generated solution. Give correct solutionarrow_forwardK Problem 23 of 24 Submit Draw the product of the reaction shown below at physiological pH (pH = 7.4). Ignore inorganic byproducts. S O 1. NH3, 2. HCN 3. H+, H₂O, A Select to Drawarrow_forwardGive detailed mechanism Solution with explanation needed..don't give Ai generated solutionarrow_forward
- Please correct answer and don't used hand raitingarrow_forward14. Draw all of the products expected for the following reaction. Circle the products expected to predominate when the reaction is heated to 40 °C. EXPLAIN your choice. (12 points) HBr ? Br -11arrow_forwardPlease correct answer and don't used hand raitingarrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning





