
a)
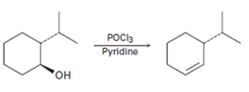
Interpretation:
A mechanism using curved arrows, for the conversion of alcohol given into the corresponding alkene by treating with POCl3, is to be proposed. The regiochemistry of the elimination is also to be explained.
Concept introduction:
The dehydration of alcohols to
To propose:
A mechanism using curved arrows, for the conversion of alcohol given into the corresponding alkene by treating with POCl3 is to be proposed.
To explain:
The regiochemistry of the elimination reaction.
b)
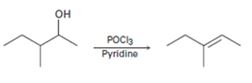
Interpretation:
A mechanism using curved arrows, for the conversion of alcohol given into the corresponding alkene by treating with POCl3, is to be proposed. The regiochemistry of the elimination is also to be explained.
Concept introduction:
The dehydration of alcohols to alkenes in the presence of POCl3 follows E2 mechanism. The –OH group is converted into a better leaving group dichlorophosphate, –OPOCl2, when treated with POCl3. Pyridine used as solvent is also a base and it removes a proton arranged anti to the leaving group from the β carbon (requirement for E2 mechanism). The removal of the proton and –OPOCl2 occurs simultaneously in a single step to yield the alkene as the product.
To propose:
A mechanism using curved arrows, for the conversion of alcohol given into the corresponding alkene by treating with POCl3, is to be proposed.
To explain:
The regiochemistry of the elimination reaction.
c)
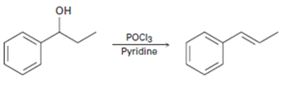
Interpretation:
A mechanism using curved arrows, for the conversion of alcohol given into the corresponding alkene by treating with POCl3, is to be proposed. The regiochemistry of the elimination is also to be explained.
Concept introduction:
The dehydration of alcohols to alkenes in the presence of POCl3 follows E2 mechanism. The –OH group is converted into a better leaving group dichlorophosphate, –OPOCl2, when treated with POCl3. Pyridine used as solvent is also a base and it removes a proton arranged anti to the leaving group from the β carbon (requirement for E2 mechanism). The removal of the proton and –OPOCl2 occurs simultaneously in a single step to yield the alkene as the product.
To propose:
A mechanism using curved arrows, for the conversion of alcohol given into the corresponding alkene by treating with POCl3, is to be proposed.
To explain:
The regiochemistry of the elimination reaction.
Trending nowThis is a popular solution!

Chapter 17 Solutions
OWLv2 with Student Solutions Manual eBook, 4 terms (24 months) Printed Access Card for McMurry's Organic Chemistry, 9th
- Calculate the ionization energy of He+ and Li²+ ions in their ground states. Thannnxxxxx sirrr Ahehehehehejh27278283-4;*; shebehebbw $+$;$-;$-28283773838 hahhehdvaarrow_forwardPlleeaasseee solllveeee question 3 andd thankss sirr, don't solve it by AI plleeaasseee don't use AIarrow_forwardCalculate the chemical shifts in 13C and 1H NMR for 4-chloropropiophenone ? Write structure and label hydrogens and carbonsarrow_forward
- 4. Read paragraph 4.15 from your textbook, use your calculated lattice energy values for CuO, CuCO3 and Cu(OH)2 an explain thermal decomposition reaction of malachite: Cu2CO3(OH)2 →2CuO + H2O + CO2 (3 points)arrow_forwardPlease sirrr soollveee these parts pleaseeee and thank youuuuuarrow_forwardIII O Organic Chemistry Using wedges and dashes in skeletal structures Draw a skeletal ("line") structure for each of the molecules below. Be sure your structures show the important difference between the molecules. key O O O O O CHON Cl jiii iiiiiiii You can drag the slider to rotate the molecules. Explanation Check Click and drag to start drawing a structure. Q Search X G ©2025 McGraw Hill LLC. All Rights Reserved. Terms of Use F 3 W C 3/5arrow_forward
- 3. Use Kapustinskii's equation and data from Table 4.10 in your textbook to calculate lattice energies of Cu(OH)2 and CuCO3 (4 points)arrow_forward2. Copper (II) oxide crystalizes in monoclinic unit cell (included below; blue spheres 2+ represent Cu²+, red - O²-). Use Kapustinski's equation (4.5) to calculate lattice energy for CuO. You will need some data from Resource section of your textbook (p.901). (4 points) CuOarrow_forwardWhat is the IUPAC name of the following compound? OH (2S, 4R)-4-chloropentan-2-ol O (2R, 4R)-4-chloropentan-2-ol O (2R, 4S)-4-chloropentan-2-ol O(2S, 4S)-4-chloropentan-2-olarrow_forward
