
Concept explainers
(a)
Interpretation: The IUPAC and common name (if any) of the following compound should be determined:
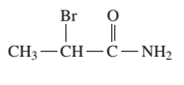
Concept Introduction: An organic compound in which carboxy
The reaction which results in the formation of amide along with water on heating acids with
So, in order to give the IUPAC name to the amides, the rules for naming carboxylic acid is followed and -oic acid of the carboxylic acid is replaced by amide.
In order to give the name to the amide group, the following steps are followed:
- The parent (longest)
alkane chain is named as for carboxylic acids. - The -oic acid in the name is changed to -amide.
- The numbering of the chain is done in such a way that amide group and substituents gets the smaller number.
- N-alkyl is used to show each alkyl group bonded to -N atom in the name for secondary and tertiary amides.
For number of carbons atoms chain, the prefix is given as:
Carbon-1 meth
Carbon-2 eth
Carbon-3 prop
Carbon-4 but
Carbon-5 pent
Carbon-6 hex
Carbon-7 hept
Carbon-8 oct
Carbon-9 non
Carbon-10 dec
In order to write the common name of the amides, the common of acids are written from which the amide has been formed by replacing -oic acid in name from -amide.
(b)
Interpretation: The IUPAC and common name (if any) of the following compound should be determined:
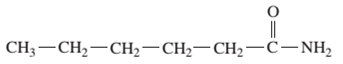
Concept Introduction: An organic compound in which carboxy functional group that is -COOH is bonded to the carbon atom is said to be a carboxylic acid. The general formula for carboxylic acid is RCOOH or RCO2H. When -OH (hydroxyl group) of the carboxylic acid is replaced by nitrogen (-N) then it results in the formation of an amide.
The reaction which results in the formation of amide along with water on heating acids with amine or ammonia is said to be amidation.
So, in order to give the IUPAC name to the amides, the rules for naming carboxylic acid is followed and -oic acid of the carboxylic acid is replaced by amide.
In order to give the name to the amide group, the following steps are followed:
- The parent (longest) alkane chain is named as for carboxylic acids.
- The -oic acid in the name is changed to -amide.
- The numbering of the chain is done in such a way that amide group and substituents gets the smaller number.
- N-alkyl is used to show each alkyl group bonded to -N atom in the name for secondary and tertiary amides.
For number of carbons atoms chain, the prefix is given as:
Carbon-1 meth
Carbon-2 eth
Carbon-3 prop
Carbon-4 but
Carbon-5 pent
Carbon-6 hex
Carbon-7 hept
Carbon-8 oct
Carbon-9 non
Carbon-10 dec
In order to write the common name of the amides, the common of acids are written from which the amide has been formed by replacing -oic acid in name from -amide.
(c)
Interpretation: The IUPAC and common name (if any) of the following compound should be determined:
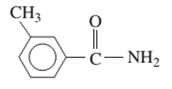
Concept Introduction: An organic compound in which carboxy functional group that is -COOH is bonded to the carbon atom is said to be a carboxylic acid. The general formula for carboxylic acid is RCOOH or RCO2H. When -OH (hydroxyl group) of the carboxylic acid is replaced by nitrogen (-N) then it results in the formation of an amide.
The reaction which results in the formation of amide along with water on heating acids with amine or ammonia is said to be amidation.
So, in order to give the IUPAC name to the amides, the rules for naming carboxylic acid is followed and -oic acid of the carboxylic acid is replaced by amide.
In order to give the name to the amide group, the following steps are followed:
- The parent (longest) alkane chain is named as for carboxylic acids.
- The -oic acid in the name is changed to -amide.
- The numbering of the chain is done in such a way that amide group and substituents gets the smaller number.
- N-alkyl is used to show each alkyl group bonded to -N atom in the name for secondary and tertiary amides.
For number of carbons atoms chain, the prefix is given as:
Carbon-1 meth
Carbon-2 eth
Carbon-3 prop
Carbon-4 but
Carbon-5 pent
Carbon-6 hex
Carbon-7 hept
Carbon-8 oct
Carbon-9 non
Carbon-10 dec
In order to write the common name of the amides, the common of acids are written from which the amide has been formed by replacing -oic acid in name from -amide.
Want to see the full answer?
Check out a sample textbook solution
Chapter 17 Solutions
Pearson eText Basic Chemistry -- Instant Access (Pearson+)
- if the answer is no reaction than state that and please hand draw!arrow_forward"I have written solutions in text form, but I need experts to rewrite them in handwriting from A to Z, exactly as I have written, without any changes."arrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward
- Please correct answer and don't used hand raitingarrow_forwardreciprocal lattices rotates along with the real space lattices of the crystal. true or false?arrow_forwardDeducing the reactants of a Diels-Alder reaction vn the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? ? Δ O If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. • If your answer is no, check the box under the drawing area instead. Click and drag to start drawing a structure. Product can't be made in one step. Explanation Checkarrow_forward
- Predict the major products of the following organic reaction: Δ ? Some important notes: • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. Explanation Check Click and drag to start drawing a structure. Larrow_forward> Can the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? ? Δ • If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. If your answer is no, check the box under the drawing area instead. Explanation Check Click and drag to start drawing a structure. Х © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accesarrow_forwardPredict the major products of the following organic reaction: O O + A ? Some important notes: • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. Explanation Check Click and drag to start drawing a structure. eserved. Terms of Use | Privacy Center >arrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





