
Pearson eText Basic Chemistry -- Instant Access (Pearson+)
6th Edition
ISBN: 9780135765982
Author: Karen Timberlake, William Timberlake
Publisher: PEARSON+
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 17.6, Problem 50PP
Write the IUPAC and common name (if any) for each of the following
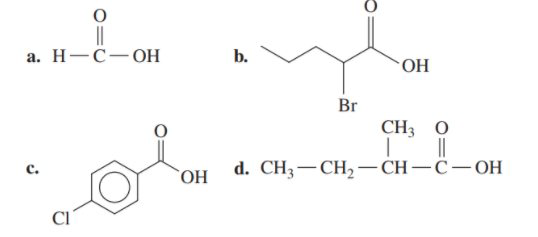
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Seee the attached ima
Please see the attached image.
Please see the attached image.
Chapter 17 Solutions
Pearson eText Basic Chemistry -- Instant Access (Pearson+)
Ch. 17.1 - Prob. 1PPCh. 17.1 - Prob. 2PPCh. 17.1 - Prob. 3PPCh. 17.1 - Prob. 4PPCh. 17.1 - Prob. 5PPCh. 17.1 - Prob. 6PPCh. 17.1 - Prob. 7PPCh. 17.1 - Prob. 8PPCh. 17.1 - Prob. 9PPCh. 17.1 - Prob. 10PP
Ch. 17.1 - Prob. 11PPCh. 17.1 - Draw the condensed structural formula for each of...Ch. 17.1 - Prob. 13PPCh. 17.1 - Prob. 14PPCh. 17.1 - Prob. 15PPCh. 17.1 - Prob. 16PPCh. 17.1 - Prob. 17PPCh. 17.1 - Prob. 18PPCh. 17.2 - Prob. 19PPCh. 17.2 - Identify each of the following as an alkane,...Ch. 17.2 - Prob. 21PPCh. 17.2 - Prob. 22PPCh. 17.2 - Prob. 23PPCh. 17.2 - Prob. 24PPCh. 17.2 - Draw the condensed structural formula for the...Ch. 17.2 - Prob. 26PPCh. 17.2 - Prob. 27PPCh. 17.2 - Prob. 28PPCh. 17.2 - Prob. 29PPCh. 17.2 - Prob. 30PPCh. 17.2 - Prob. 31PPCh. 17.2 - Prob. 32PPCh. 17.3 - Prob. 33PPCh. 17.3 - Prob. 34PPCh. 17.3 - Prob. 35PPCh. 17.3 - Draw the line-angle formula for each of the...Ch. 17.4 - Prob. 37PPCh. 17.4 - Prob. 38PPCh. 17.4 - Prob. 39PPCh. 17.4 - Write the common name for each of the following:...Ch. 17.4 - Draw the condensed structural and line-angle...Ch. 17.4 - Draw the condensed structural and line-angle...Ch. 17.5 - Write the common name for each of the following:Ch. 17.5 - Write the common name for each of the following:Ch. 17.5 - Prob. 45PPCh. 17.5 - Prob. 46PPCh. 17.5 - Prob. 47PPCh. 17.5 - Draw the condensed structural formula for a and b...Ch. 17.6 - Prob. 49PPCh. 17.6 - Write the IUPAC and common name (if any) for each...Ch. 17.6 - Prob. 51PPCh. 17.6 - Prob. 52PPCh. 17.6 - Prob. 53PPCh. 17.6 - Prob. 54PPCh. 17.6 - Prob. 55PPCh. 17.6 - Prob. 56PPCh. 17.6 - Prob. 57PPCh. 17.6 - Write the IUPAC and common names, if any, for each...Ch. 17.6 - Draw the condensed structural formulas for a and b...Ch. 17.6 - Draw the condensed structural formulas for a and b...Ch. 17.7 - Write the common name for each of the following:...Ch. 17.7 - Prob. 62PPCh. 17.7 - Prob. 63PPCh. 17.7 - Prob. 64PPCh. 17.7 - Prob. 65PPCh. 17.7 - Prob. 66PPCh. 17.7 - Prob. 67PPCh. 17.7 - Prob. 68PPCh. 17.7 - Prob. 69PPCh. 17.7 - Prob. 70PPCh. 17.7 - Prob. 71PPCh. 17.7 - Prob. 72PPCh. 17 - The chapter sections to review are shown in...Ch. 17 - The chapter sections to review are shown in...Ch. 17 - Prob. 75UTCCh. 17 - Prob. 76UTCCh. 17 - Prob. 77APPCh. 17 - Prob. 78APPCh. 17 - Prob. 79APPCh. 17 - Prob. 80APPCh. 17 - Prob. 81APPCh. 17 - Prob. 82APPCh. 17 - Prob. 83APPCh. 17 - Prob. 84APPCh. 17 - Classify each of the following according to its...Ch. 17 - Classify each of the following according to its...Ch. 17 - Name each of the following aromatic compounds:...Ch. 17 - Prob. 88APPCh. 17 - Prob. 89APPCh. 17 - Draw the structural formula for each of the...Ch. 17 - Prob. 91APPCh. 17 - Prob. 92APPCh. 17 - Draw the condensed structural formula for each of...Ch. 17 - Draw the condensed structural formula for each of...Ch. 17 - Write the IUPAC name for each of the following:...Ch. 17 - Write the IUPAC name for each of the following:...Ch. 17 - Draw the condensed structural formulas for a and b...Ch. 17 - Prob. 98APPCh. 17 - Prob. 99APPCh. 17 - Prob. 100APPCh. 17 - Prob. 101APPCh. 17 - Draw the condensed structural formula for each of...Ch. 17 - Prob. 103APPCh. 17 - Prob. 104APPCh. 17 - Prob. 105CPCh. 17 - Prob. 106CPCh. 17 - The following problems are related to the topics...Ch. 17 - Prob. 108CPCh. 17 - The following problems are related to the topics...Ch. 17 - Prob. 110CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- V Biological Macromolecules Drawing the Haworth projection of an aldose from its Fischer projection Draw a Haworth projection of a common cyclic form of this monosaccharide: H C=O HO H HO H H OH CH₂OH Explanation Check Click and drag to start drawing a structure. Xarrow_forwardComplete the mechanismarrow_forwardComplete the mechanismarrow_forward
- 8 00 6 = 10 10 Decide whether each of the molecules in the table below is stable, in the exact form in which it is drawn, at pH = 11. If you decide at least one molecule is not stable, then redraw one of the unstable molecules in its stable form below the table. (If more than unstable, you can pick any of them to redraw.) Check OH stable HO stable Ounstable unstable O OH stable unstable OH 80 F6 F5 stable Ounstable X Save For Later Sub 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy C ཀྭ་ A F7 매 F8 F9 4 F10arrow_forwardJust try completing it and it should be straightforward according to the professor and TAs.arrow_forwardThe grading is not on correctness, so if you can just get to the correct answers without perfectionism that would be great. They care about the steps and reasoning and that you did something. I asked for an extension, but was denied the extension.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY