
Concept explainers
(a)
Interpretation:
From the given reaction it should be showed that how the product is prepared from the starting material.
Concept Introduction:
Oxidation: If electrons are moved from a species during a
Generally, oxidation of
(b)
Interpretation:
From the given reaction it should be showed that how the product is prepared from the starting material.
Concept Introduction:
Reduction: If electrons are gained to a species during a chemical reaction. The species which gets electrons are said to be reduced.
Generally, reduction of primary alcohol results to give aldehyde.
Aldehyde (except formaldehyde) in presence of Grignard Reagent results in formation of secondary alcohol.
Oxidation: If electrons are moved from a species during a chemical reaction. The species whose electrons are removed is said to be oxidized.
Generally, oxidation of primary alcohol forms aldehyde and secondary alcohol forms ketone.
(c)
Interpretation:
From the given reaction it should be showed that how the product prepared from the starting material.
Concept introduction:
Reduction: If electrons are gained to a species during a chemical reaction. The species which gets electrons are said to be reduced.
Reaction for aldehydes and ketones in presence of primary
The carbonyl group of a
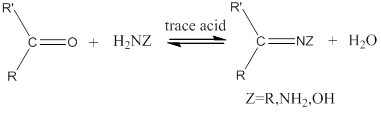
Oxidation: If electrons are moved from a species during a chemical reaction. The species whose electrons are removed is said to be oxidized.
Generally, oxidation of primary alcohol forms aldehyde and secondary alcohol forms ketone.
(d)
Interpretation:
From the given reaction it should be showed that how the product is prepared from the starting material.
Concept introduction:
Reduction: If electrons are gained to a species during a chemical reaction. The species which gets electrons are said to be reduced.
Oxidation: If electrons are moved from a species during a chemical reaction. The species whose electrons are removed is said to be oxidized.
Generally, oxidation of primary alcohol forms aldehyde and secondary alcohol forms ketone.
(e)
Interpretation:
From the given reaction it should be showed that how the product is prepared from the starting material.
Concept introduction:
Reduction: If electrons are gained to a species during a chemical reaction. The species which gets electrons are said to be reduced.
Oxidation: If electrons are moved from a species during a chemical reaction. The species whose electrons are removed is said to be oxidized.
Generally, oxidation of primary alcohol forms aldehyde and secondary alcohol forms ketone.
Ketone in presence of Grignard Reagent results to form tertiary alcohol.
Want to see the full answer?
Check out a sample textbook solution
Chapter 17 Solutions
EBK ORGANIC CHEMISTRY
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





