
Concept explainers
Draw the structure for each of the following:
- a. isobutyraldehyde
- b. 4-hexenaI
- c. diisopentyl
ketone - d. 3-methylcyclohexanone
- e. 2.4-pentanedione
- f. 4-bromo-3-heptanone
- g. γ-bromocaproaldehyde
- h. 2-ethylcyclopentanecarbaldehyde
- i. 4-methyl-5-oxohexanal
(a)
Interpretation:
The structure of Isobutyraldehyde has to be drawn.
Answer to Problem 53P
The structure of Isobutyraldehyde is given below:
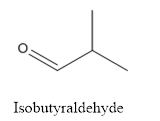
Explanation of Solution
The compound has aldehyde group that is attached to the isobutyl group.
The structure of isobutyraldehyde is as follows.

(b)
Interpretation:
The structure of 4-hexenal has to be drawn.
Answer to Problem 53P
The structure of 4-hexenal is given below:
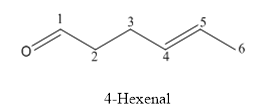
Explanation of Solution
The compound has aldehyde functional group and double bond at the 4th position of the compound.
The structure is as follows.

(c)
Interpretation:
The structure of diisopentyl ketone has to be drawn.
Answer to Problem 53P
The structure of diisopentyl ketone is given below:
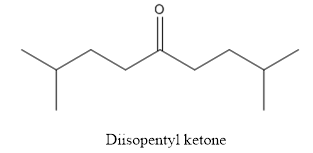
Explanation of Solution
The compound has ketone functional group. Two isopentyl groups are attached to the keto group.
The structure of diisopentyl ketone is as follows.

(d)
Interpretation:
The structure of 3-methylcyclohexanone has to be drawn.
Answer to Problem 53P
The structure of 3-methylcyclohexanoneis given below:
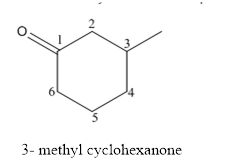
Explanation of Solution
The compound has ketone functional and group and it is called as cyclohexanone and it has one methyl group at 3rd position of the ring.
The structure of 3-methyl cyclohexanone is as follows.

(e)
Interpretation:
The structure of 2,4-pentanedione has to be drawn.
Answer to Problem 53P
The structure of 2,4-pentanedione is given below:
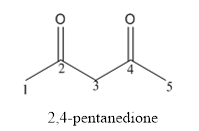
Explanation of Solution
The compound has two keto functional groups and these are attached to the pentane carbon chain.

(f)
Interpretation:
The structure of 4-bromo-3-heptanone has to be drawn.
Answer to Problem 53P
The structure of 4-bromo-3-heptanone is given below:
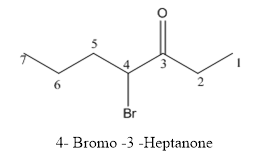
Explanation of Solution
The compound has keto functional group and it is attached to the heptanes carbon chain. At the 4th position of the carbon chain has bromine attachment.

(g)
Interpretation:
The structure of
Answer to Problem 53P
The structure of
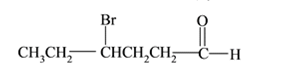
Explanation of Solution
Compound has aldehyde functional group and at the
The structure of
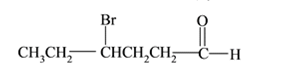
(h)
Interpretation:
The structure of 2-ethylcyclopentanecarbaldehyde has to be drawn.
Answer to Problem 53P
The structure of 2-ethylcyclopentanecarbaldehyde is given below:
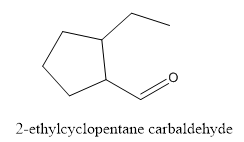
Explanation of Solution
In this compound, pentane ring has ethyl group and aldehyde functional group.
The structure of 2-ethylcyclopentane carbaldehyde.

(i)
Interpretation:
The structure of 4-methyl-5-oxohexanal has to be drawn.
Answer to Problem 53P
The structure of 4-methyl-5-oxohexanal is givenbelow:
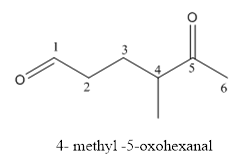
Explanation of Solution
The compound has aldehyde functional group at the 5th position of the carbon chain as keto group it is named as oxo-because it is referred to an attachment.
The structure of 4- methyl-5-oxohexanal.

Want to see more full solutions like this?
Chapter 17 Solutions
EBK ORGANIC CHEMISTRY
- 18 Question (1 point) Draw the line structure form of the given partially condensed structure in the box provided. :ÖH HC HC H2 ΙΩ Н2 CH2 CH3 CH3 partially condensed formarrow_forwardsomeone else has already submitted the same question on here and it was the incorrect answer.arrow_forwardThe reaction: 2NO2(g) ⇌ N2O4(g) is an exothermic reaction, ΔH=-58.0 kJ/molrxn at 0°C the KP is 58.If the initial partial pressures of both NO2(g) and N2O4(g) are 2.00 atm:A) Is the reaction at equilibrium? If not, what is the value of Q? B) Which direction will the reaction go to reach equilibrium? C) Use an ICE table to find the equilibrium pressures.arrow_forward
- The dissociation of the weak acid, nitrous acid, HNO2, takes place according to the reaction: HNO2 (aq) ⇌ H+(aq) + NO2–(aq) K=7.2 X 10-4 When 1.00 mole of HNO2 is added to 1.00 L of water, the H+ concentration at equilibrium is 0.0265 M.A) Calculate the value of Q if 1.00 L of water is added? B) How will reaction shift if 1.00 L of water is added?arrow_forwardSuppose a certain copolymer elastomeric material “styrene-butadiene rubber”) contains styrene ("S") monomers –(C8H8)– and butadiene ("B") monomers –(C4H6)– and that their numerical ratio S:B = 1:8. What is the mass ratio mS:mB of the two monomers in the material? What is the molecular mass M of a macromolecule of this copolymer with degree of polymerization n = 60,000? Data: AC = 12.01 u, AH = 1.008 u.arrow_forwardLab Questions from Lab: Gravimetric Determination of Calcium as CaC2O4•H2O What is the purpose of the methyl red indicator? Why does a color change to yellow tell you that the reaction is complete? Why is the precipitate rinsed with ice-cold water in step 4? Why not room temperature or hot water? Why is it important that the funnels be placed in a desiccator before weighing (steps 1 and 5)?arrow_forward
- What mass of ethylene glycol, HOCH2CH2OH, Mustbe added to 5.50 kg of water to antifreeze that would work for the car radiator to -10.0 degrees celcius? MM (g/mol): 62.07arrow_forwardWhat is the molarity of a 0.393 m glucose solution if its density is 1.16 g/mL? MM glucose 180.2 g/molarrow_forwardThe rate constant for the decay of a radioactive element is 2.28 × 10⁻³ day⁻¹. What is the half-life of this element in days?arrow_forward
- Handwritten pleasearrow_forwardChoose the best reagents to complete the following reaction. i H A B 1. CH3CH2Na 2. H3O+ 1. CH3CH2MgBr 2. H3O+ 1. CH3MgBr Q C 2. H3O+ 1. H3O+ D 2. CH3MgBr 00 OH Q E CH³MgBrarrow_forwardThe kinetics of a gas phase reaction of the form A → Products results in a rate constant of 0.00781 M/min. For this reaction, the initial concentration of A is 0.501 M. What is the half-life for this reaction?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





