
Organic Chemistry
11th Edition
ISBN: 9781118133576
Author: T. W. Graham Solomons, Craig Fryhle
Publisher: Wiley, John & Sons, Incorporated
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 17, Problem 5Q
Complete the following synthesis.
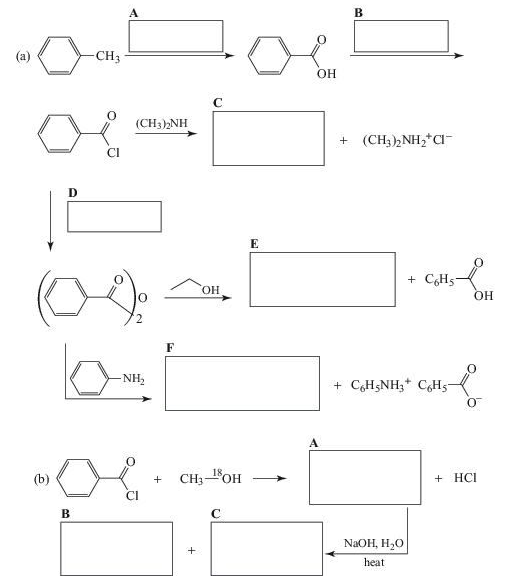
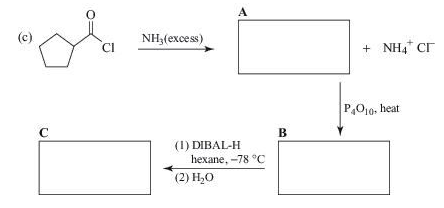
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
I need help with the following question
For CARS, which statement is not true regarding its advantages?
a) Contrast signal based on vibrational characteristics, no need for fluorescent tagging.
b) Stronger signals than spontaneous Raman.
c) Suffers from fluorescence interference, because CARS signal is at high frequency.
d) Faster, more efficient imaging for real-time analysis.
e) Higher resolution than spontaneous Raman microscopy.
Draw the major product of the Claisen condensation reaction between two molecules of this ester. Ignore
inorganic byproducts.
Incorrect, 5 attempts remaining
1. NaOCH3/CH3OH
2. Acidic workup
Select to Draw
O
Incorrect, 5 attempts remaining
The total number of carbons in the parent chain is incorrect. Review the reaction conditions including starting materials and/or
intermediate structures and recount the number of carbon atoms in the parent chain of your structure.
OK
Chapter 17 Solutions
Organic Chemistry
Ch. 17 - Practice Problem 17.1 Give an IUPAC systematic...Ch. 17 - Prob. 2PPCh. 17 - PRACTICE PROBLEM
17.3 Which acid of each pair...Ch. 17 - Practice Problem 17.3 Write structural formulas...Ch. 17 - Practice Problem 17.4
Show how each of the...Ch. 17 - Practice Problem 17.5
Show how you could prepare...Ch. 17 - Practice Problem 17.6
(a) Which of the carboxylic...Ch. 17 - Prob. 8PPCh. 17 - Prob. 9PPCh. 17 - Practice Problem 17.9
Esters can also be...
Ch. 17 - Prob. 11PPCh. 17 - Prob. 12PPCh. 17 - Practice Problem 17.12
What products would you...Ch. 17 - Practice Problem 17.13 (a) Provide the reagents...Ch. 17 - Prob. 15PPCh. 17 - Practice Problem 17.15 Using decarboxylation...Ch. 17 - Practice Problem 17.16 Diacyl peroxides, ,...Ch. 17 - Prob. 18PCh. 17 - Give an IUPAC systematic or common name for each...Ch. 17 - Prob. 20PCh. 17 - Prob. 21PCh. 17 - 17.21 What major organic product would you expect...Ch. 17 - Prob. 23PCh. 17 - Prob. 24PCh. 17 - Prob. 25PCh. 17 - Prob. 26PCh. 17 - 17.26 What products would you expect to obtain...Ch. 17 - Write structural formulas for the major organic...Ch. 17 - 17.28 Indicate reagents that would accomplish each...Ch. 17 - Write structural formulas for the major organic...Ch. 17 - Prob. 31PCh. 17 - Prob. 32PCh. 17 - Prob. 33PCh. 17 - 17.33 On heating,...Ch. 17 - Prob. 35PCh. 17 - Prob. 36PCh. 17 - 17.36 Show how pentanoic acid can be prepared from...Ch. 17 - 17.37 The active ingredient of the insect...Ch. 17 - Prob. 39PCh. 17 - Prob. 40PCh. 17 - Give stereochemical formulas for compounds AQ:...Ch. 17 - 17.41 -Glyceraldehyde can be transformed into...Ch. 17 - Prob. 43PCh. 17 - 17.44 Cantharidin is a powerful vesicant that can...Ch. 17 - Prob. 45PCh. 17 - 17.44 Given here are the NMR spectra and carbonyl...Ch. 17 - Compound X (C7H12O4) is insoluble in aqueous...Ch. 17 - 17.45 Compound Y dissolves slowly when warmed...Ch. 17 - Prob. 49PCh. 17 - Prob. 50PCh. 17 - 17.52 Starting with 1-naphthol, suggest an...Ch. 17 - Suggest a synthesis of ibuprofen (Section 5.11)...Ch. 17 - Prob. 53PCh. 17 - Prob. 54PCh. 17 - Prob. 1LGPCh. 17 - Prob. 2LGPCh. 17 - Prob. 3LGPCh. 17 - Prob. 4LGPCh. 17 - Prob. 1QCh. 17 - 17.2 Which of the following would yield...Ch. 17 - 17.3 Which reagent would serve as the basis for a...Ch. 17 - Prob. 4QCh. 17 - Complete the following synthesis.Ch. 17 - 17.6 Which of these acids would undergo...
Additional Science Textbook Solutions
Find more solutions based on key concepts
One isomer of methamphetamine is the addictive illegal drug known as crank. Another isomer is a medicine for si...
Campbell Essential Biology (7th Edition)
Write an equation that uses the products of photosynthesis as reactants and the reactants of photosynthesis as ...
Campbell Biology in Focus (2nd Edition)
What distinguishes the mass spectrum of 2,2-dimethylpropane from the mass spectra of pentane and isopentane?
Organic Chemistry (8th Edition)
When you rub your cold hands together, the friction between them results in heat that warms your hands. Why doe...
Anatomy & Physiology (6th Edition)
11. Birds and mammals are both endothermic, and both have four-chambered hearts. Most reptiles are ectothermic ...
Campbell Biology: Concepts & Connections (9th Edition)
16. In a large metropolitan hospital, cells from newborn babies are collected and examined microscopically over...
Genetic Analysis: An Integrated Approach (3rd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Using a cell of known pathlength b = 1.25115 x 10-3 cm, a water absorption spectrum was measured. The band at 1645 cm-1, assigned to the O-H bending, showed an absorbance, A, of 1.40. a) Assuming that water density is 1.00 g/mL, calculate the water molar concentration c (hint: M= mole/L) b) Calculate the molar absorptivity, a, of the 1645 cm-1 band c) The transmitted light, I, can be written as I= Ioexp(-xb), where x is the absorption coefficient (sometimes designated as alpha), Io is the input light, and b is the cell pathlength. Prove that x= (ln10)*x*c d) Calculate x for the 1645 cm-1 bandarrow_forwardConvert 1.38 eV into wavelength (nm) and wavenumber (cm-1) (c = 2.998 x 108 m/s; h = 6.626 x 10-34 J*s).arrow_forwardCan you help me understand the CBC method on metal bridging by looking at this problem?arrow_forward
- A partir de Aluminio y Co(NO3)2ꞏ6H2O, indicar las reacciones a realizar para obtener Azul de Thenard (Al2CoO4).arrow_forwardTo obtain Thenard Blue (Al2CoO4), the following reaction is correct (performed in an oven):Al(OH)3 + Co(OH)2 → Al2CoO4 + 4 H2Oarrow_forwardProblem 38 can u explain and solve thanks april 24arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
How to Design a Total Synthesis; Author: Chemistry Unleashed;https://www.youtube.com/watch?v=9jRfAJJO7mM;License: Standard YouTube License, CC-BY