
(a)
Interpretation:
The reaction in which
Concept introduction:
Grignard reagents are
Answer to Problem 17.45AP
The complete reaction between
![]()
Explanation of Solution
The given reaction is shown below.
![]()
Figure 1
The complete reaction is shown below.
![]()
Figure 2
The hydrolysis of Grignard reagent is shown in Figure 2 in the presence of a solvent
Therefore, the product formed is shown in Figure 2.
The complete reaction between
(b)
Interpretation:
The reaction between
Concept introduction:
Grignard reagents are organometallic compounds which are prepared using alkyl halides in the presence of magnesium metal in dry ether. These reagents act as strong nucleophiles and bases.
Answer to Problem 17.45AP
The complete reaction between
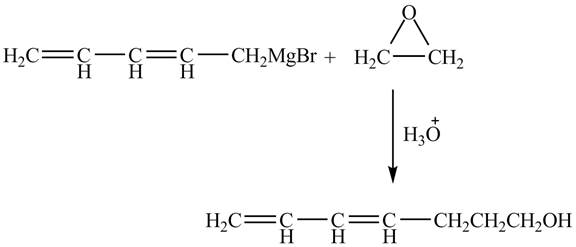
Explanation of Solution
The given reaction is shown below.
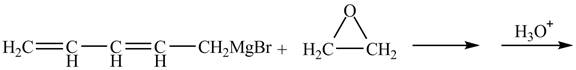
Figure 3
The complete reaction is shown below.
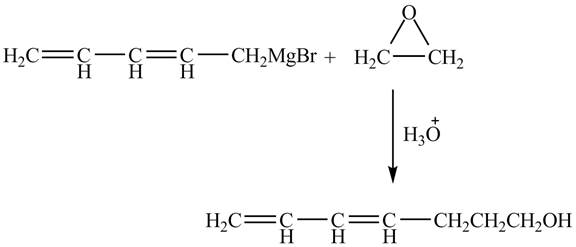
Figure 4
The Grignard reagent on treatment with epoxides in the presence of
Therefore, the product formed is shown in Figure 4.
The complete reaction between
(c)
Interpretation:
The reaction between
Concept introduction:
Nucleophiles are electron-rich species. The nucleophilic substitution reactions are the reactions in which nucleophile attacks the electrophilic center and eliminates another group.
Answer to Problem 17.45AP
The complete reaction between
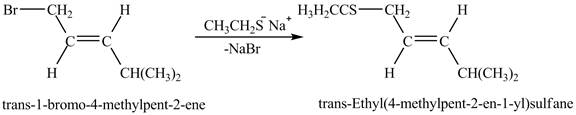
Explanation of Solution
The given reaction is shown below.
![]()
Figure 5
The complete reaction is shown below.
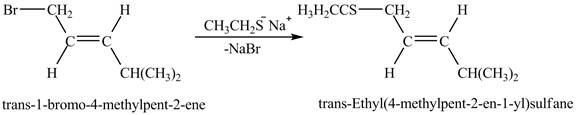
Figure 6
The above figure shows the reaction between ethyl sulfide ion and
The complete reaction between
(d)
Interpretation:
The reaction between
Concept introduction:
Nucleophiles are electron-rich species. The nucleophilic substitution reactions are the reactions in which nucleophile attacks the electrophilic center and eliminates another group.
Answer to Problem 17.45AP
The complete reaction between
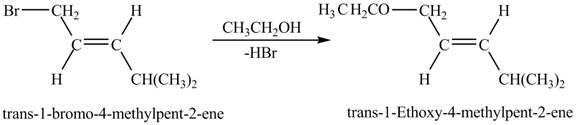
Explanation of Solution
The given reaction is shown below.

Figure 7
The complete reaction is shown below.
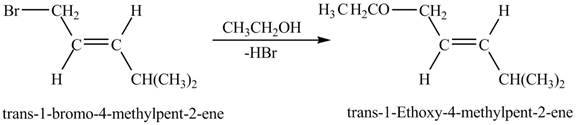
Figure 8
Figure 8 shows the reaction between
The complete reaction between
(e)
Interpretation:
The reaction of a given compound with NBS is to be completed.
Concept introduction:
Cyclic alkenes on reaction with N-Bromosuccinimide (NBS) forms allyl bromide, that is, bromine is substituted at the allylic position. NBS is a rich source of free radical of
Answer to Problem 17.45AP
The complete reaction of a given compound with NBS is shown below.
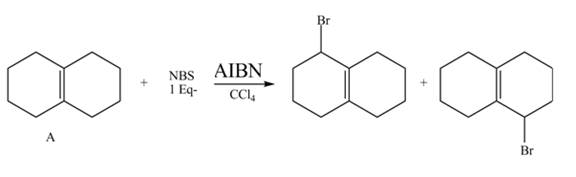
Explanation of Solution
The given reaction is shown below.
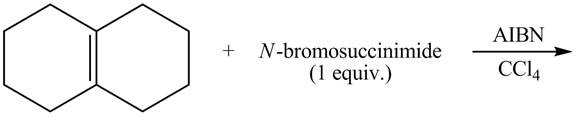
Figure 9
The complete reaction is shown below.

Figure 10
The NBS is used for allylic bromination that is it substitutes
Therefore, the products formed are shown in Figure 10.
The complete reaction of given compound with NBS is shown in Figure 10.
(f)
Interpretation:
The reaction between
Concept introduction:
Cyclic alkenes on reaction with N-Bromosuccinimide (NBS) forms allyl bromide, that is, bromine is substituted at the allylic position. NBS is a rich source of free radical of
Answer to Problem 17.45AP
The complete reaction between

Explanation of Solution
The given reaction is shown below.
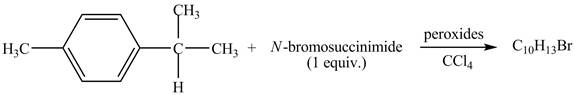
Figure 11
The complete reaction is shown below.

Figure 12
The molecular formula of the product is
Due to the addition of NBS, benzylic bromination of
Therefore, the product formed is shown in Figure 12.
The complete reaction between
(g)
Interpretation:
The complete reaction in which
Concept introduction:
The elimination reaction of alkyl halide,
Answer to Problem 17.45AP
The complete reaction in which
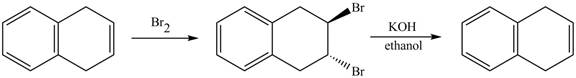
Explanation of Solution
The given reaction is shown below.
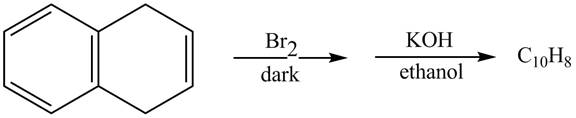
Figure 13
The complete reaction is shown below.

Figure 14
The addition of
Therefore, the product formed is shown in Figure 14.
The complete reaction in which
(h)
Interpretation:
The reaction between
Concept introduction:
When an alkene reacts with water in the acidic medium the reaction follows Markovnikov rule which states that the negative part of the reagent attacks the carbon with the least number of hydrogen atoms attached to it.
Answer to Problem 17.45AP
The complete reaction between
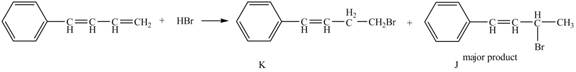
Explanation of Solution
The given reaction is shown below.
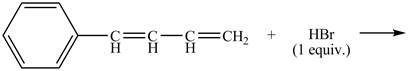
Figure 15
The complete reaction is shown below.

Figure 16
The compound,
Therefore, the products formed are shown in Figure 16.
The complete reaction between
(i)
Interpretation:
The reaction between
Concept introduction:
The substance which gets easily reduced is termed as a strong oxidizing agent. It is also defined as the substances which oxidize other substances by accepting their electrons. Examples of strong oxidizing agents are potassium permanganate, hydrogen peroxide and many more.
Answer to Problem 17.45AP
The complete reaction between
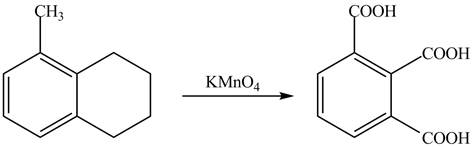
Explanation of Solution
The given reaction is shown below.
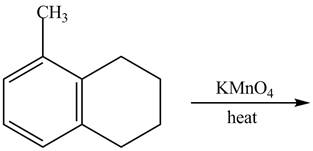
Figure 17
The complete reaction is shown below.

Figure 18
The compound,
Therefore, the product formed is shown in Figure 18.
The complete reaction between
Want to see more full solutions like this?
Chapter 17 Solutions
EBK ORGANIC CHEMISTRY STUDY GUIDE AND S
- Indicate the products obtained when tert-butylbenzene reacts with a sulfonitric acid mixture (HNO3 + H2SO4). Indicate the majority if necessary.arrow_forwardIndicate the products obtained when acetophenone reacts with a sulfonitric acid mixture (HNO3 + H2SO4). Indicate the majority if necessary.arrow_forwardIndicate the products obtained from the reaction of N-(4-methylphenyl)acetamide with a sulfonitric acid mixture (H2SO4 + HNO3). Indicate the majority if necessary.arrow_forward
- Indicate the products obtained from the reaction of 4-(trifluoromethyl)benzonitrile with a sulfonitric mixture (H2SO4 + HNO3). Indicate the majority if necessary.arrow_forwardIndicate the products obtained in the reaction of p-Toluidine with a sulfonitric acid mixture (H2SO4 + HNO3). Indicate the majority if necessary.arrow_forwardIndicate the products obtained from the reaction of 4-methylbenzonitrile with a sulfonitric acid mixture (H2SO4 + HNO3). Indicate the majority if necessary.arrow_forward
- Indicate the products obtained from the reaction of 2-nitrophenol with a sulfonitric acid mixture (H2SO4 + HNO3). Indicate the majority if necessary.arrow_forwardIn organic chemistry, what is the correct name for the mixture H2SO4 + HNO3 used in reactions: sulphonitric mixture or sulfonitric mixture?arrow_forwardFormulate the products obtained by reacting p-toluidine with a sulfonate mixture. Indicate the majority if necessary.arrow_forward
- Consider this organic reaction: OH Draw the major products of the reaction in the drawing area below. If there won't be any major products, because this reaction won't happen at a significant rate, check the box under the drawing area instead. Click and drag to start drawing a structure. x 0: の Carrow_forwardExplain the reasons for a compound's greater or lesser reactivity toward electrophilic aromatic substitution. Give reasons.arrow_forwardDraw the products of a reaction of the following alkyle chloride, shown below in the 3D ball and stick model with NaSCH3. Ignore inorganic byproducts. In the figure, a gray ball indicates a carbon atom a white ball indicates a hydrogen atom anda agreen ball indicated a chlorine atomarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
