
FUND.OF GEN CHEM CHAP 1-13 W/ACCESS
16th Edition
ISBN: 9781323406038
Author: McMurry
Publisher: PEARSON C
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 17, Problem 17.43AP
Give systematic names for the following
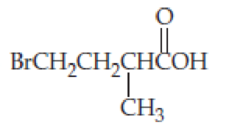
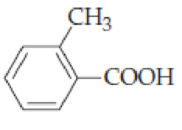
- (a) (CH3CH2)3CCOOH
- (b) CH3(CH2)5COOH
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
At a pH equal to the isoelectric point (pl) of alanine, the net charge of alanine is zero. Two structures can be drawn that have a
net charge of zero, but the predominant form of alanine at its pl is zwitterionic.
CH3
H,N
CH3
**
H¸N-C
H
Zwitterionic
H
Uncharged
OH
Select statements that explain why alanine is predominantly zwitterionic at its pl.
pk of alanine's amino group is more than its pl.
pk of alanine's carboxyl group is more than its pl.
PK of alanine's carboxyl group is less than its pl.
pk of alanine's amino group is less than its pl.
Correct Answer
What fraction of alanine is in the completely uncharged form at its pl?
1 in 2.2 × 107
1 in 1.6 × 10²
1 in 4680
1 in 9460
How does a voltage-gated sodium channel work? Specifically, how and why does a change in voltage trigger their opening? Please be detailed
When sodium ions enter a neuron during depolarization, they trigger the opening of additional voltage-gated sodium channels nearby, creating a positive feedback loop where the influx of sodium ions further depolarizes the membrane, causing even more sodium channels to open and allowing more sodium ions to enter the cell, thus sustaining the depolarization process until the action potential peaks. But how and why exactly does the influx of sodium ions trigger more sodium channels to let in more sodium? Please explain
Chapter 17 Solutions
FUND.OF GEN CHEM CHAP 1-13 W/ACCESS
Ch. 17.1 - Identify the following molecules as a carboxylic...Ch. 17.1 - Prob. 17.2PCh. 17.1 - Prob. 17.3PCh. 17.1 - Prob. 17.4PCh. 17.1 - Prob. 17.5PCh. 17.1 - Prob. 17.6PCh. 17.1 - In the following pairs of compounds, which would...Ch. 17.1 - Write both condensed and line structures for (a)...Ch. 17.1 - Prob. 17.9PCh. 17.1 - Draw structures corresponding to these names: (a)...
Ch. 17.1 - Prob. 17.11PCh. 17.1 - Prob. 17.12PCh. 17.1 - Prob. 17.13KCPCh. 17.2 - Salsalate, which is an ester formed by the...Ch. 17.2 - Prob. 17.2CIAPCh. 17.2 - Prob. 17.3CIAPCh. 17.2 - Prob. 17.14PCh. 17.2 - Prob. 17.15PCh. 17.2 - Prob. 17.16PCh. 17.3 - Prob. 17.17PCh. 17.3 - Raspberry oil contains an ester that is made by...Ch. 17.3 - Prob. 17.19PCh. 17.3 - Prob. 17.20PCh. 17.3 - Prob. 17.21PCh. 17.4 - If a bottle of aspirin tablets has the aroma of...Ch. 17.4 - Prob. 17.23PCh. 17.4 - What carboxylic acids and amines result from...Ch. 17.5 - Prob. 17.25PCh. 17.5 - Prob. 17.26KCPCh. 17.6 - Prob. 17.27PCh. 17.6 - Prob. 17.28PCh. 17.6 - Prob. 17.4CIAPCh. 17.6 - Prob. 17.5CIAPCh. 17.6 - Prob. 17.29PCh. 17 - Prob. 17.30UKCCh. 17 - Prob. 17.31UKCCh. 17 - One phosphorylated form of glycerate is...Ch. 17 - Prob. 17.33UKCCh. 17 - Prob. 17.34UKCCh. 17 - Prob. 17.35UKCCh. 17 - Prob. 17.36UKCCh. 17 - For the following compounds, give the systematic...Ch. 17 - Write the equation for the ionization of hexanoic...Ch. 17 - Prob. 17.39APCh. 17 - Prob. 17.40APCh. 17 - Prob. 17.41APCh. 17 - Give systematic names for the following carboxylic...Ch. 17 - Give systematic names for the following carboxylic...Ch. 17 - Prob. 17.44APCh. 17 - Prob. 17.45APCh. 17 - Draw structures corresponding to the following...Ch. 17 - Draw structures corresponding to the following...Ch. 17 - Malic acid, a dicarboxylic acid found in apples,...Ch. 17 - Prob. 17.49APCh. 17 - Prob. 17.50APCh. 17 - Prob. 17.51APCh. 17 - Prob. 17.52APCh. 17 - Prob. 17.53APCh. 17 - Give systematic names for the following structures...Ch. 17 - Give systematic names for the following structures...Ch. 17 - Prob. 17.56APCh. 17 - Prob. 17.57APCh. 17 - Give systematic names for the following structures...Ch. 17 - Give systematic names for the following structures...Ch. 17 - Prob. 17.60APCh. 17 - What compounds are produced from hydrolysis of...Ch. 17 - Procaine, a local anesthetic whose hydrochloride...Ch. 17 - Prob. 17.63APCh. 17 - Lactones are cyclic esters in which the carboxylic...Ch. 17 - When both the carboxylic acid and the amine are in...Ch. 17 - LSD (lysergic acid diethylamide), a semisynthetic...Ch. 17 - Prob. 17.67APCh. 17 - Prob. 17.68APCh. 17 - Prob. 17.69APCh. 17 - Prob. 17.70APCh. 17 - Prob. 17.71APCh. 17 - Prob. 17.72APCh. 17 - Prob. 17.73APCh. 17 - Prob. 17.74APCh. 17 - Prob. 17.75APCh. 17 - Three amide isomers, N,N-dimethylformamide,...Ch. 17 - Prob. 17.77CPCh. 17 - Prob. 17.78CPCh. 17 - Mention at least two simple chemical tests by...Ch. 17 - Prob. 17.80CPCh. 17 - Name the following compounds.Ch. 17 - Each of the following materials has an ester that...Ch. 17 - Prob. 17.83GPCh. 17 - Prob. 17.84GP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the predominant form of glutamic acid at pH = 8.4. The pKa of the side chain is 4.1. Include proper stereochemistry. HO H2N OH pH = 8.4arrow_forwardHow would I draw this?arrow_forwardCalculate the standard change in Gibbs free energy, AGrxn, for the given reaction at 25.0 °C. Consult the table of thermodynamic properties for standard Gibbs free energy of formation values. NH,Cl(s) →NH; (aq) + C1 (aq) AGrxn -7.67 Correct Answer Determine the concentration of NH+ (aq) if the change in Gibbs free energy, AGrxn, for the reaction is -9.27 kJ/mol. 6.49 [NH+] Incorrect Answer kJ/mol Marrow_forward
- What are some topics of interest that neurotoxicologists study? For example, toxin-induced seizures, brain death, and such along those lines?arrow_forwardCould you help me with the explanation of the answer to exercise 15, chapter 1 of Lehinger Question Nombramiento de estereoisómeros con dos carbonos quirales utilizando el sistema RS(R,R)El isómero del metilfenidato (Ritalin) se utiliza para tratar el trastorno por déficit de atención con hiperactividad (TDAH).(S,S)El isómero es un antidepresivo. Identifique los dos carbonos quirales en la siguiente estructura. ¿Es este el(R,R)o el(S,S)¿isómero? Dibuja el otro isómero. Nombramiento de estereoisómeros con dos carbonos quirales utilizando el sistema RS(R,R)El isómero del metilfenidato (Ritalin) se utiliza para tratar el trastorno por déficit de atención con hiperactividad (TDAH).(S,S)El isómero es un antidepresivo.arrow_forwardThe reaction A+B → C + D AG°' = -7.3 kcal/mol can be coupled with which of the following unfavorable reactions to drive it forward? A. EFG+HAG° = 5.6 kcal/mol. B. J+KZ+A AG° = 2.3 kcal/mol. C. P+RY+DAG° = 8.2 kcal/mol. D. C + T → V + W AG°' = -5.9 kcal/mol. E. AN→ Q+KAG°' = 4.3 kcal/mol.arrow_forward
- What would be the toxicological endpoints for neurotoxicity?arrow_forwardWhat are "endpoints" in toxicology exactly? Please give an intuitive easy explanationarrow_forwardFura-2 Fluorescence (Arbitrary Unit) 4500 4000 3500 3000 2500 2000 1500 1000 500 [Ca2+]=2970nM, 25°C [Ca2+] 2970nM, 4°C [Ca2+]=0.9nM, 25°C [Ca2+] = 0.9nM, 4°C 0 260 280 300 340 360 380 400 420 440 Wavelength (nm) ← < The figure on the LHS shows the excitation spectra of Fura-2 (Em = 510 nm) in 2 solutions with two different Ca2+ ion concentration as indicated. Except for temperature, the setting for excitation & signal acquisition was identical.< ப a) The unit in Y-axis is arbitrary (unspecified). Why? < < b) Compare & contrast the excitation wavelength of the Isosbestic Point of Fura-2 at 25 °C & 4 °C. Give a possible reason for the discrepancy. < c) The fluorescence intensity at 25 °C & 4 °C are different. Explain why with the concept of electronic configuration. <arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College
Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College




Biology Today and Tomorrow without Physiology (Mi...
Biology
ISBN:9781305117396
Author:Cecie Starr, Christine Evers, Lisa Starr
Publisher:Cengage Learning

Anatomy & Physiology
Biology
ISBN:9781938168130
Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark Womble
Publisher:OpenStax College

Macromolecules | Classes and Functions; Author: 2 Minute Classroom;https://www.youtube.com/watch?v=V5hhrDFo8Vk;License: Standard youtube license