
ORGANIC CHEMISTRY
5th Edition
ISBN: 9781259977596
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 17, Problem 17.36P
The purine heterocycle occurs commonly in the structure of DNA.
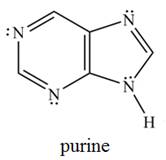
a. How is each
b. In what type of orbital does each lone pair on a
c. How many
d. Why is purine aromatic?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Viscosity of a liquid related to the activation energy.
Vibrational contributions to internal energy and heat capacity1) are temperature independent2) are temperature dependent
The approximation of calculating the partition function by integration instead of the summation of all the energy terms can only be done if the separation of the energy levels is much smaller than the product kT. Explain why.
Chapter 17 Solutions
ORGANIC CHEMISTRY
Ch. 17 - Prob. 17.1PCh. 17 - Problem 17.2 What orbitals are used to form the...Ch. 17 - Problem-17.3. Give the IUPAC name for each...Ch. 17 - Prob. 17.4PCh. 17 - Problem-17.5 What is the structure of propofol,...Ch. 17 - Problem 17.6 What is the structure of a compound...Ch. 17 - Problem 17.7 How many NMR signals does each...Ch. 17 - Prob. 17.8PCh. 17 - Prob. 17.9PCh. 17 - Prob. 17.10P
Ch. 17 - Prob. 17.11PCh. 17 - Prob. 17.12PCh. 17 - Prob. 17.13PCh. 17 - Problem 17.14 Januvia, the trade name for...Ch. 17 - Prob. 17.15PCh. 17 - Problem 17.16 Rank the following compounds in...Ch. 17 - Problem 17.17 Draw the seven resonance structures...Ch. 17 - Prob. 17.18PCh. 17 - Prob. 17.19PCh. 17 - Prob. 17.20PCh. 17 - Prob. 17.21PCh. 17 - Problem 17.22 How many NMR signals does ...Ch. 17 - 17.23 Name each compound and state how many lines...Ch. 17 - Prob. 17.24PCh. 17 - Prob. 17.25PCh. 17 - Prob. 17.26PCh. 17 - 17.27 Give the IUPAC name for each compounds.
a....Ch. 17 - 17.28 Draw a structure corresponding to each...Ch. 17 - 17.29 a. Draw the 14 constitutional isomers of...Ch. 17 - Prob. 17.30PCh. 17 - Prob. 17.31PCh. 17 - Prob. 17.32PCh. 17 - 17.33 Label each compound as aromatic,...Ch. 17 - Prob. 17.34PCh. 17 - 17.35 Pentalene, azulene, and heptalene are...Ch. 17 - 17.36 The purine heterocycle occurs commonly in...Ch. 17 - Prob. 17.37PCh. 17 - 17.38
How many electrons does C contain?
How...Ch. 17 - Prob. 17.39PCh. 17 - 17.40 Explain the observed rate of reactivity of...Ch. 17 - 17.41 Draw a stepwise mechanism for the following...Ch. 17 - Prob. 17.42PCh. 17 - 17.43 Draw additional resonance structures for...Ch. 17 - Prob. 17.44PCh. 17 - Prob. 17.45PCh. 17 - 17.46 Which compound in each pair is the stronger...Ch. 17 - 17.47 Treatment of indene with forms its...Ch. 17 - Prob. 17.48PCh. 17 - 17.49 Draw the conjugate bases of pyrrole and...Ch. 17 - 17.50 a. Explain why protonation of pyrrole occurs...Ch. 17 - Prob. 17.51PCh. 17 - Prob. 17.52PCh. 17 - 17.53 How many signals does each compound...Ch. 17 - 17.54 Which of the diethylbenzene isomers (ortho,...Ch. 17 - 17.55 Propose a structure consistent with each...Ch. 17 - 17.56 Propose a structure consistent with each...Ch. 17 - 17.57 Thymol (molecular formula ) is the major...Ch. 17 - 17.58 You have a sample of a compound of molecular...Ch. 17 - 17.59 Explain why tetrahydrofuran has a higher...Ch. 17 - 17.60 Rizatriptan (trade name Maxalt) is a...Ch. 17 - 17.61 Zolpidem (trade name Ambien) promotes the...Ch. 17 - 17.62 Answer the following questions about...Ch. 17 - 17.63 Stanozolol is an anabolic steroid that...Ch. 17 - Prob. 17.64PCh. 17 - 17.65 Use the observed data to decide whether C...Ch. 17 - Prob. 17.66PCh. 17 - Prob. 17.67PCh. 17 - Prob. 17.68PCh. 17 - 17.69 Although benzene itself absorbs at in its ...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Explain the meaning of: the electron partition function is equal to the degeneracy of the ground state.arrow_forward28. For each of the following species, add charges wherever required to give a complete, correct Lewis structure. All bonds and nonbonded valence electrons are shown. a. b. H H H H H :0-C-H H H H-C-H C. H H d. H-N-0: e. H H-O H-O H B=0 f. H—Ö—Ñ—Ö—H Norton Private Barrow_forwardAt 0oC and 1 atm, the viscosity of hydrogen (gas) is 8.55x10-5 P. Calculate the viscosity of a gas, if possible, consisting of deuterium. Assume that the molecular sizes are equal.arrow_forward
- Indicate the correct option for the velocity distribution function of gas molecules:a) its velocity cannot be measured in any other way due to the small size of the gas moleculesb) it is only used to describe the velocity of particles if their density is very high.c) it describes the probability that a gas particle has a velocity in a given interval of velocitiesd) it describes other magnitudes, such as pressure, energy, etc., but not the velocity of the moleculesarrow_forwardIndicate the correct option for the velocity distribution function of gas molecules:a) its velocity cannot be measured in any other way due to the small size of the gas moleculesb) it is only used to describe the velocity of particles if their density is very high.c) it describes the probability that a gas particle has a velocity in a given interval of velocitiesd) it describes other magnitudes, such as pressure, energy, etc., but not the velocity of the moleculesarrow_forwardDraw the skeletal structure of the alkane 4-ethyl-2, 2, 5, 5- tetramethylnonane. How many primary, secondary, tertiary, and quantenary carbons does it have?arrow_forward
- Electronic contribution to the heat capacity at constant volume A) is always zero B) is zero, except for excited levels whose energy is comparable to KT C) equals 3/2 Nk D) equals Nk exp(BE)arrow_forwardPlease correct answer and don't used hand raitingarrow_forwardCalculate the packing factor of CaTiO3. It has a perovskite structure. Data: ionic radii Co²+ = 0.106 nm, Ti4+ = 0.064 nm, O² = 0.132 nm; lattice constant is a = 2(rTi4+ + ro2-). Ca2+ 02- T14+ Consider the ions as rigid spheres. 1. 0.581 or 58.1% 2. -0.581 or -58.1 % 3. 0.254 or 25.4%arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY