
Organic Chemistry
5th Edition
ISBN: 9780078021558
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 17, Problem 17.2P
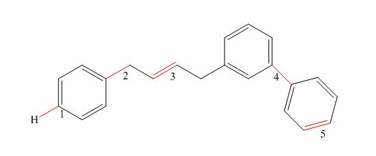
What orbitals are used to form the labeled bonds in the following molecule? Of the labeled
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Show mechanism..don't give Ai generated solution
Don't used Ai solution
Show work. Don't give Ai generated solution
Chapter 17 Solutions
Organic Chemistry
Ch. 17 - Prob. 17.1PCh. 17 - Problem 17.2 What orbitals are used to form the...Ch. 17 - Problem-17.3. Give the IUPAC name for each...Ch. 17 - Prob. 17.4PCh. 17 - Problem-17.5 What is the structure of propofol,...Ch. 17 - Problem 17.6 What is the structure of a compound...Ch. 17 - Problem 17.7 How many NMR signals does each...Ch. 17 - Prob. 17.8PCh. 17 - Prob. 17.9PCh. 17 - Prob. 17.10P
Ch. 17 - Prob. 17.11PCh. 17 - Prob. 17.12PCh. 17 - Prob. 17.13PCh. 17 - Problem 17.14 Januvia, the trade name for...Ch. 17 - Prob. 17.15PCh. 17 - Problem 17.16 Rank the following compounds in...Ch. 17 - Problem 17.17 Draw the seven resonance structures...Ch. 17 - Prob. 17.18PCh. 17 - Prob. 17.19PCh. 17 - Prob. 17.20PCh. 17 - Prob. 17.21PCh. 17 - Problem 17.22 How many NMR signals does ...Ch. 17 - 17.23 Name each compound and state how many lines...Ch. 17 - Prob. 17.24PCh. 17 - Prob. 17.25PCh. 17 - Prob. 17.26PCh. 17 - 17.27 Give the IUPAC name for each compounds.
a....Ch. 17 - 17.28 Draw a structure corresponding to each...Ch. 17 - 17.29 a. Draw the 14 constitutional isomers of...Ch. 17 - Prob. 17.30PCh. 17 - Prob. 17.31PCh. 17 - Prob. 17.32PCh. 17 - 17.33 Label each compound as aromatic,...Ch. 17 - Prob. 17.34PCh. 17 - 17.35 Pentalene, azulene, and heptalene are...Ch. 17 - 17.36 The purine heterocycle occurs commonly in...Ch. 17 - Prob. 17.37PCh. 17 - 17.38
How many electrons does C contain?
How...Ch. 17 - Prob. 17.39PCh. 17 - 17.40 Explain the observed rate of reactivity of...Ch. 17 - 17.41 Draw a stepwise mechanism for the following...Ch. 17 - Prob. 17.42PCh. 17 - 17.43 Draw additional resonance structures for...Ch. 17 - Prob. 17.44PCh. 17 - Prob. 17.45PCh. 17 - 17.46 Which compound in each pair is the stronger...Ch. 17 - 17.47 Treatment of indene with forms its...Ch. 17 - Prob. 17.48PCh. 17 - 17.49 Draw the conjugate bases of pyrrole and...Ch. 17 - 17.50 a. Explain why protonation of pyrrole occurs...Ch. 17 - Prob. 17.51PCh. 17 - Prob. 17.52PCh. 17 - 17.53 How many signals does each compound...Ch. 17 - 17.54 Which of the diethylbenzene isomers (ortho,...Ch. 17 - 17.55 Propose a structure consistent with each...Ch. 17 - 17.56 Propose a structure consistent with each...Ch. 17 - 17.57 Thymol (molecular formula ) is the major...Ch. 17 - 17.58 You have a sample of a compound of molecular...Ch. 17 - 17.59 Explain why tetrahydrofuran has a higher...Ch. 17 - 17.60 Rizatriptan (trade name Maxalt) is a...Ch. 17 - 17.61 Zolpidem (trade name Ambien) promotes the...Ch. 17 - 17.62 Answer the following questions about...Ch. 17 - 17.63 Stanozolol is an anabolic steroid that...Ch. 17 - Prob. 17.64PCh. 17 - 17.65 Use the observed data to decide whether C...Ch. 17 - Prob. 17.66PCh. 17 - Prob. 17.67PCh. 17 - Prob. 17.68PCh. 17 - 17.69 Although benzene itself absorbs at in its ...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- this is an organic chemistry question please answer accordindly!! please post the solution draw the figures on a paper please hand drawn and post, please answer EACH part till the end and dont just provide wordy explanations, please draw them on a paper and post clearly!! answer the full question with all details EACH PART CLEARLY please thanks!! im reposting this please solve all parts and draw it not just word explanations!!arrow_forwardA mixture of 0.412 M C12, 0.544 M F2, and 0.843 M CIF is enclosed in a vessel and heated to 2500 K. C12(g) + F2(g )2CIF(g) Kc = 20.0 at 2500 K Calculate the equilibrium concentration of each gas at 2500 K. [C12] = M [F2] = M [ CIF] =arrow_forwardShow reaction mechanism with explanation. don't give Ai generated solutionarrow_forward
- Don't used Ai solutionarrow_forwardthis is an organic chemistry question please answer accordindly!! please post the solution draw the figures and post, answer the question in a very simple and straight forward manner thanks!!!!! please answer EACH part till the end and dont just provide wordy explanations wherever asked for structures or diagrams, please draw them on a paper and post clearly!! answer the full question with all details EACH PART CLEARLY please thanks!! im reposting this kindly solve all parts and draw it not just word explanations!!arrow_forwardPlease correct answer and don't used hand raitingarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY