
Principles of General, Organic, Biological Chemistry
2nd Edition
ISBN: 9780073511191
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 17, Problem 17.27UKC
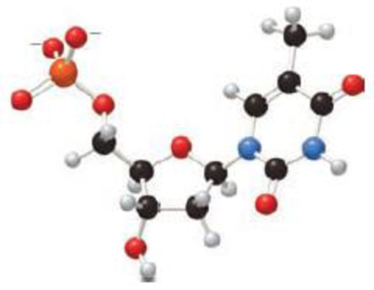
(a) Identify the base and monosaccharide in the following
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
C
Predict the major products of this organic reaction.
Be sure you use wedge and dash bonds when necessary, for example to distinguish between major products with different stereochemistry.
: ☐
+
x
G
C
RCO₂H
Click and drag to start
drawing a structure.
Fill in the blanks by selecting the appropriate term from below:
For a process that is non-spontaneous and that favors products at equilibrium, we know that a) ΔrG∘ΔrG∘ _________, b) ΔunivSΔunivS _________, c) ΔsysSΔsysS _________, and d) ΔrH∘ΔrH∘ _________.
Highest occupied molecular orbital
Lowest unoccupied molecular orbital
Label all nodes and regions of highest and lowest electron density for both orbitals.
Chapter 17 Solutions
Principles of General, Organic, Biological Chemistry
Ch. 17.1 - Prob. 17.1PCh. 17.1 - Draw the structure of guanosine. Classify the...Ch. 17.1 - Prob. 17.3PCh. 17.1 - Give the name that corresponds to each...Ch. 17.1 - Which nucleic acid (DNA or RNA) contains each of...Ch. 17.1 - Label each statement about the compound...Ch. 17.1 - Identify each component as a base, nucleoside, or...Ch. 17.2 - Draw the structure of a dinucleotide formed by...Ch. 17.2 - Label the 5' end and the 3' end in each...Ch. 17.2 - Label each statement about the polynucleotide...
Ch. 17.3 - Write the complementary strand for each of the...Ch. 17.4 - What is the sequence of a newly synthesized DNA...Ch. 17.6 - Prob. 17.13PCh. 17.6 - Prob. 17.14PCh. 17.11 - Prob. 17.24PCh. 17 - Label each statement as pertaining to DNA, RNA, or...Ch. 17 - (a) Identify the base and monosaccharide in the...Ch. 17 - (a) Identify the base and monosaccharide in the...Ch. 17 - Consider the given dinucleotide. a. Identify the...Ch. 17 - Answer Problem 17.29 for the following...Ch. 17 - Fill in the codon, anticodon, or amino acid needed...Ch. 17 - Fill in the codon, anticodon, or amino acid needed...Ch. 17 - What is the difference between a gene and a...Ch. 17 - Prob. 17.38APCh. 17 - List three structural differences between DNA and...Ch. 17 - List three structural similarities in DNA and RNA.Ch. 17 - Prob. 17.41APCh. 17 - Prob. 17.42APCh. 17 - Give the name, abbreviation, or structure of each...Ch. 17 - Give the name, abbreviation, or structure of each...Ch. 17 - Classify each molecule as a nucleoside or...Ch. 17 - Draw the structure of the deoxyribonucleotide...Ch. 17 - Draw the structure of the ribonucleotide formed by...Ch. 17 - Describe in detail the DNA double helix with...Ch. 17 - Describe in detail the DNA double helix with...Ch. 17 - Write the sequence of the complementary strand of...Ch. 17 - Write the sequence of the complementary strand of...Ch. 17 - What is the sequence of the mRNA molecule...Ch. 17 - What is the sequence of the mRNA molecule...Ch. 17 - Considering each nucleotide sequence in an mRNA...Ch. 17 - Considering each nucleotide sequence in an mRNA...Ch. 17 - What is the difference between a point mutation...Ch. 17 - Consider the following mRNA sequence: CUU CAG CAC....Ch. 17 - Consider the following mRNA sequence: ACC UUA CGA....
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Relative Intensity Part VI. consider the multi-step reaction below for compounds A, B, and C. These compounds were subjected to mass spectrometric analysis and the following spectra for A, B, and C was obtained. Draw the structure of B and C and match all three compounds to the correct spectra. Relative Intensity Relative Intensity 20 NaоH 0103 Br (B) H2504 → (c) (A) 100- MS-NU-0547 80 40 20 31 10 20 100- MS2016-05353CM 80 60 100 MS-NJ-09-3 80 60 40 20 45 J.L 80 S1 84 M+ absent राग 135 137 S2 62 164 166 11 S3 25 50 75 100 125 150 175 m/zarrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forwardDon't used hand raitingarrow_forward
- Don't used hand raitingarrow_forwardA composite material reinforced with aligned fibers, consisting of 20% by volume of silicon carbide (SiC) fibers and 80% by volume of polycarbonate (PC) matrix. The mechanical characteristics of the 2 materials are in the table. The stress of the matrix when the fiber breaks is 45 MPa. Calculate the longitudinal strength? SiC PC Elastic modulus (GPa) Tensile strength (GPa) 400 2,4 3,9 0,065arrow_forwardQuestion 2 What starting materials or reagents are best used to carry out the following reaction? 2Fe, 3Br2 ○ FeCl3 2Fe, 4Br2 O Heat and Br2 Heat and HBr Brarrow_forward
- What is/are the major product(s) of the following reaction? O AICI -Chts +arrow_forwardShown below is the major resonance structure for a molecule. Draw the second best resonance structure of the molecule. Include all non-zero formal charges. H. C H H C H :Ö: Click and drag to start drawing a structure.arrow_forwardShown below is the major resonance structure for a molecule. Draw the second best resonance structure of the molecule. Include all non-zero formal charges. H. C H H C. H H H H Click and drag to start drawing a structure. Xarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning  Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Nucleic acids - DNA and RNA structure; Author: MEDSimplified;https://www.youtube.com/watch?v=0lZRAShqft0;License: Standard YouTube License, CC-BY