
Concept explainers
(a)
Interpretation:
The electrodes have to be labelled and the ions present in the solution has to be identified.
Concept introduction:
Voltaic cell (or) galvanic cell:
Voltaic cell is an experimental setup used to generate
(a)
Explanation of Solution
Zinc electrode is labelled as anode and lead is labelled as cathode.
The ions present in the solution are Lead, nitrate and Zinc.
The electrolytic cell with labelled electrodes and ions are shown below,
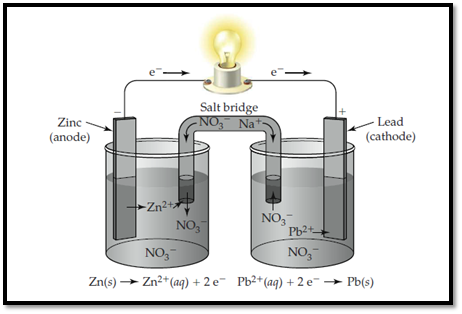
Figure 1
(b)
Interpretation:
The cathode and anode have to be labelled.
Concept introduction:
Voltaic cell (or) galvanic cell:
Voltaic cell is an experimental setup used to generate electric current through the spontaneous redox reaction. The voltaic cell is an electrochemical cell, which is converting a chemical energy into electrical energy. Cathode as the positive electrode. Anode as the negative electrode. Salt bridge is required. Ions are discharged only in cathode while anode is consumed.
(b)
Explanation of Solution
Zinc electrode is labelled as anode and lead is labelled as cathode. In anode, zinc undergoes oxidation and in cathode, lead undergoes reduction.
The electrolytic cell with labelled electrodes is shown below,
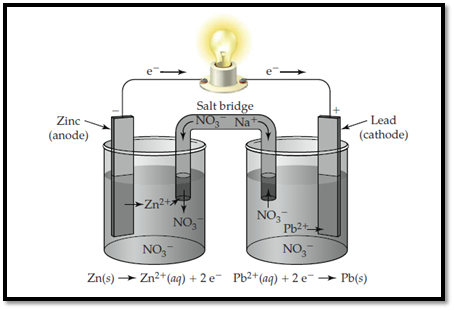
Figure 1
(c)
Interpretation:
The direction of flow of electron in the wire and ion flow in solution has to be indicated.
Concept introduction:
Voltaic cell (or) galvanic cell:
Voltaic cell is an experimental setup used to generate electric current through the spontaneous redox reaction. The voltaic cell is an electrochemical cell, which is converting a chemical energy into electrical energy. Cathode as the positive electrode. Anode as the negative electrode. Salt bridge is required. Ions are discharged only in cathode while anode is consumed.
(c)
Explanation of Solution
Zinc electrode is labelled as anode and lead is labelled as cathode. In anode, zinc undergoes oxidation and in cathode, lead undergoes reduction. The flow of ions and electrons in the solution is shown below,
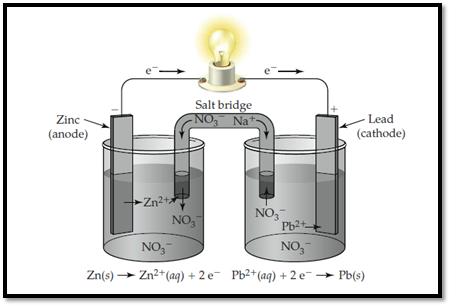
Figure 1
(d)
Interpretation:
The direction ion flow in solution and the electrolyte that is used for passage of ions has to be indicated.
Concept introduction:
Voltaic cell (or) galvanic cell:
Voltaic cell is an experimental setup used to generate electric current through the spontaneous redox reaction. The voltaic cell is an electrochemical cell, which is converting a chemical energy into electrical energy. Cathode as the positive electrode. Anode as the negative electrode. Salt bridge is required. Ions are discharged only in cathode while anode is consumed.
(d)
Explanation of Solution
Zinc electrode is labelled as anode and lead is labelled as cathode. In anode, zinc undergoes oxidation and in cathode, lead undergoes reduction. The flow of ions and electrons in the solution is shown below. The passage of ions takes place with Sodium nitrate that acts as salt bridge.
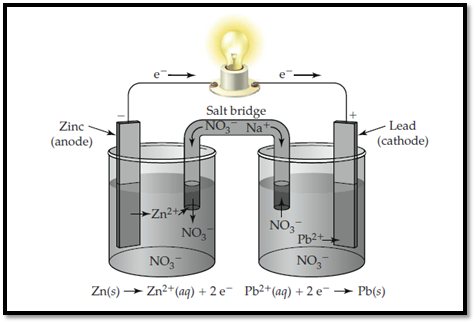
Figure 1
(e)
Interpretation:
The balance equation for the electrode and the overall cell reaction has to be given.
Concept introduction:
Voltaic cell (or) galvanic cell:
Voltaic cell is an experimental setup used to generate electric current through the spontaneous redox reaction. The voltaic cell is an electrochemical cell, which is converting a chemical energy into electrical energy. Cathode as the positive electrode. Anode as the negative electrode. Salt bridge is required. Ions are discharged only in cathode while anode is consumed.
(e)
Explanation of Solution
Zinc electrode is labelled as anode and lead is labelled as cathode. In anode, zinc undergoes oxidation and in cathode, lead undergoes reduction. The flow of ions and electrons in the solution is shown below. The passage of ions takes place with Sodium nitrate that acts as salt bridge.
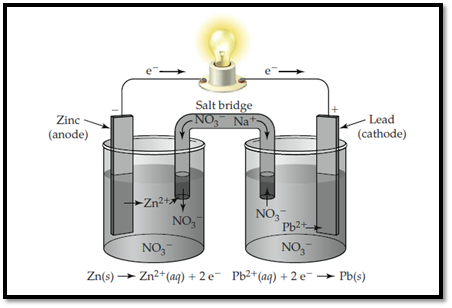
Figure 1
In anode, zinc undergoes oxidation and in cathode, lead undergoes reduction.
The reactions at the cathode and anode is given as,
The overall reaction is given by summing up the cathode and anode reactions.
The overall reaction is written as,
Want to see more full solutions like this?
Chapter 17 Solutions
General Chemistry: Atoms First
- What is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forwardIn the two chair conformations of glucose, the most stable is the one with all the OH groups in the equatorial position. Is this correct?arrow_forward
- please help me with my homeworkarrow_forwardhelparrow_forwardThe temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forward
- QUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forwarder your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning





