
Concept explainers
(a)
Interpretation:
Show how to bring out the given conversion reaction in good yield.
Concept introduction:
Alkyl or aryl magnesium halides (RMgX) are known as Grignard reagent. The Grignard reaction is an organometallic
Synthesis of Grignard reagent is shown below,
The
Addition of a Grignard reagent to carbon dioxide followed by protonation will produce
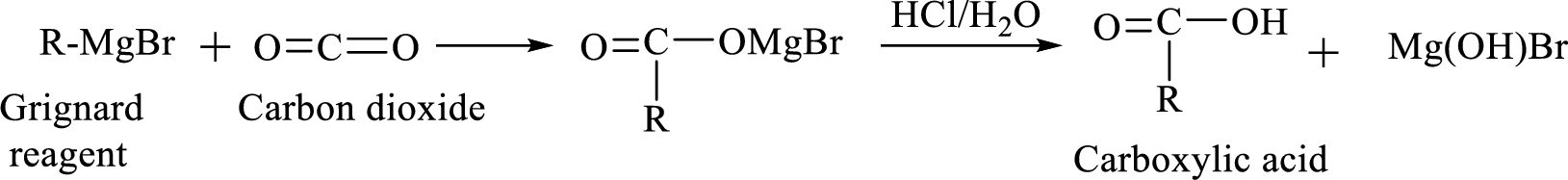
(b)
Interpretation:
Show how to bring out the given conversion reaction in good yield.
Concept introduction:
Carboxylic acid can be prepared from various ways; oxidation of
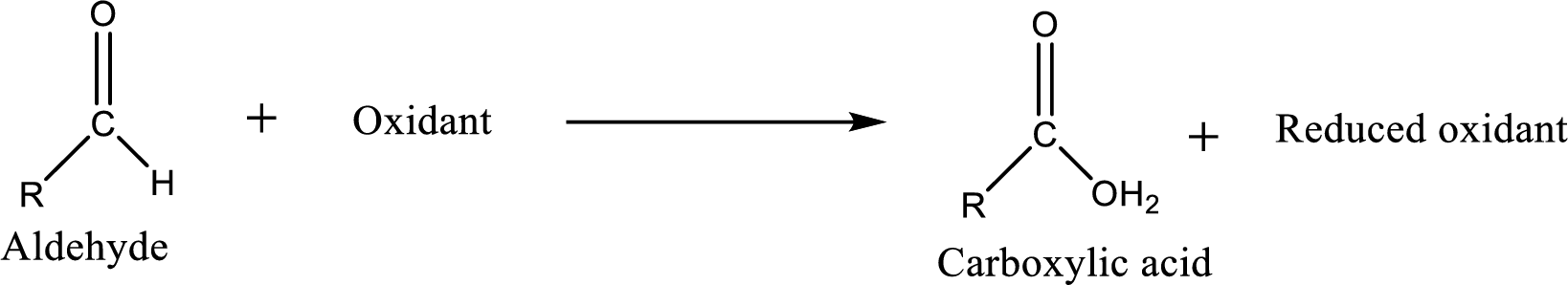
Carboxylic acid can be prepared from various ways; oxidation of aldehyde is one of the important methods to prepare carboxylic acid.
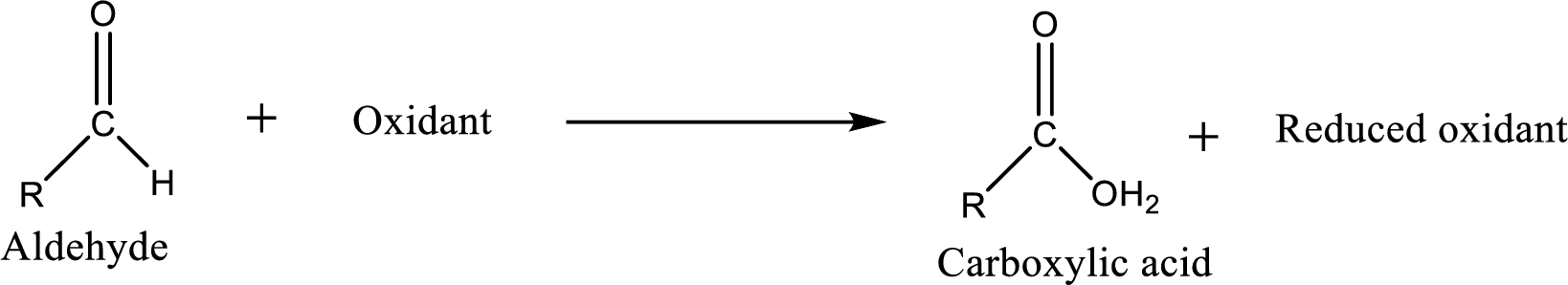
Carboxylic acid on further oxidation removes the carboxyl carbon as carbon dioxide. Depending on the reaction conditions, the oxidation state of the remaining organic structure may be higher, lower or unchanged.
Carboxylic acid can be prepared from primary alcohol by oxidation using strong oxidizing agents like chromic acid,
(c)
Interpretation:
Show how to bring out the given conversion reaction in good yield.
Concept introduction:
Carboxylic acid can be prepared from various ways; oxidation of aldehyde is one of the important methods to prepare carboxylic acid.
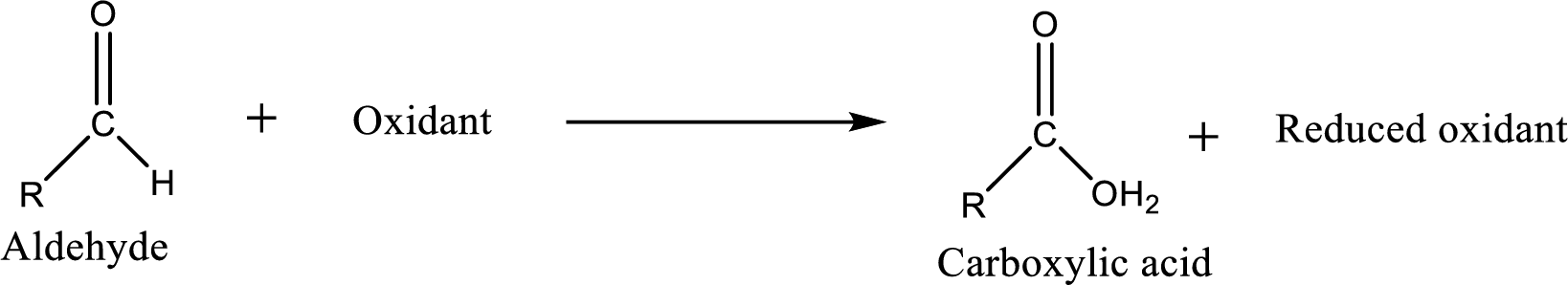
Carboxylic acid can be prepared from various ways; oxidation of aldehyde is one of the important methods to prepare carboxylic acid.
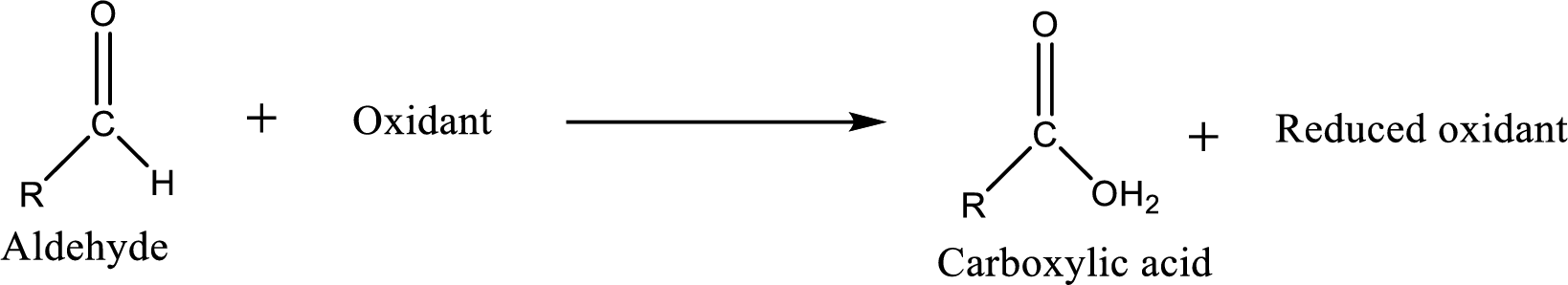
Carboxylic acid on further oxidation removes the carboxyl carbon as carbon dioxide. Depending on the reaction conditions, the oxidation state of the remaining organic structure may be higher, lower or unchanged.
Carboxylic acid can be prepared from primary alcohol by oxidation using strong oxidizing agents like chromic acid,
Trending nowThis is a popular solution!

Chapter 17 Solutions
OWLv2 with MindTap Reader, 1 term (6 months) Printed Access Card for Brown/Iverson/Anslyn/Foote's Organic Chemistry, 8th Edition
- Synthesize 2-Ethyl-3-methyloxirane from dimethyl(propyl)sulfonium iodide using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Synthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIf possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forwardSynthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Indicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forwardWe mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forwardIndicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forward
- Indicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forwardIndicate the products obtained if (E)-2-butenal and 3-oxo-butanenitrile are mixed with sodium ethoxide in ethanol.arrow_forwardQuestion 3 (4 points), Draw a full arrow-pushing mechanism for the following reaction Please draw all structures clearly. Note that this intramolecular cyclization is analogous to the mechanism for halohydrin formation. COH Br + HBr Brarrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
