
Organic Chemistry
8th Edition
ISBN: 9781337516402
Author: Brown
Publisher: Cengage
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16.8, Problem FQ
The given mechanism of transamination reaction is shown below,
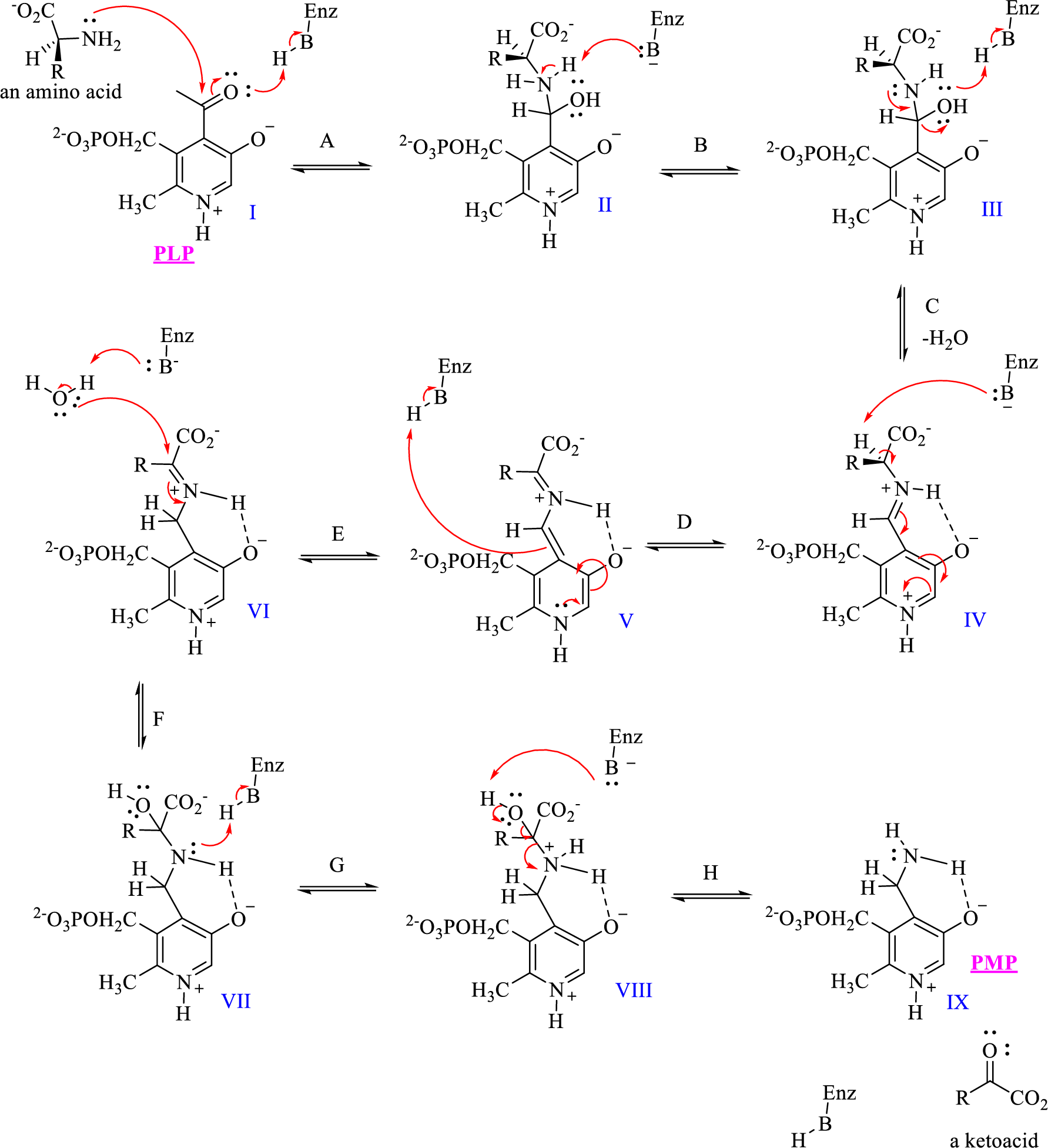
To complete the shuffling of amino groups and carbonyls, the PMP must be converted back to PLP. How would you predict that this occurs?
- 1. A separate reaction oxidizes the
amine group of PMP to analdehyde . - 2. A separate reaction hydrolyzes the amine of PMP to an aldehyde.
- 3. A different ketoacid reacts with the amine of PMP, and the entire sequence runs backward.
- 4. Either 2 or 3 could occur.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Using reaction free energy to predict equilibrium composition
Consider the following equilibrium:
N2O4 (g) 2NO2 (g)
AG⁰ = 5.4 kJ
Now suppose a reaction vessel is filled with 1.68 atm of dinitrogen tetroxide (N204) at 148. °C. Answer the following questions about this system:
rise
Under these conditions, will the pressure of N2O4 tend to rise or fall?
x10
fall
Is it possible to reverse this tendency by adding NO2?
In other words, if you said the pressure of N2O4 will tend to rise, can that
be changed to a tendency to fall by adding NO2? Similarly, if you said the
pressure of N2O4 will tend to fall, can that be changed to a tendency to
rise by adding NO2?
If you said the tendency can be reversed in the second question, calculate
the minimum pressure of NO 2 needed to reverse it.
Round your answer to 2 significant digits.
yes
no
0.42 atm
☑
5
0/5
?
مله
Ar
Homework 13 (Ch17)
Question 4 of 4 (1 point) | Question Attempt: 2 of 2
✓ 1
✓ 2
= 3
4
Time Remaining: 4:25:54
Using the thermodynamic information in the ALEKS Data tab, calculate the standard reaction free energy of the following chemical reaction:
2CH3OH (g)+302 (g) → 2CO2 (g) + 4H₂O (g)
Round your answer to zero decimal places.
☐ kJ
x10
☐
Subm
Check
2020 Hill LLC. All Rights Reserved. Terms of Use | Privacy Ce
Identifying the major species in weak acid or weak base equilibria
Your answer is incorrect.
• Row 2: Your answer is incorrect.
• Row 3: Your answer is incorrect.
• Row 6: Your answer is incorrect.
0/5
The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at
equilibrium. You can leave out water itself.
Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the
formulas of the species that will act as neither acids nor bases in the 'other' row.
You will find it useful to keep in mind that HF is a weak acid.
acids:
HF
0.1 mol of NaOH is added to
1.0 L of a 0.7M HF
solution.
bases:
0.13 mol of HCl is added to
1.0 L of a solution that is
1.0M in both HF and KF.
Exponent
other:
F
acids: HF
bases: F
other:
K
1
0,0,...
?
000
18
Ar
Chapter 16 Solutions
Organic Chemistry
Ch. 16.1 - Write the IUPAC name for each compound. Specify...Ch. 16.1 - Write structural formulas for all aldehydes with...Ch. 16.1 - Write the IUPAC name for each compound.Ch. 16.5 - Prob. 16.4PCh. 16.6 - Prob. 16.5PCh. 16.7 - Prob. 16.6PCh. 16.7 - Write a mechanism for the acid-catalyzed...Ch. 16.8 - Prob. 16.8PCh. 16.8 - The given mechanism of transamination reaction is...Ch. 16.8 - The given mechanism of transamination reaction is...
Ch. 16.8 - The given mechanism of transamination reaction is...Ch. 16.8 - Prob. DQCh. 16.8 - Prob. EQCh. 16.8 - The given mechanism of transamination reaction is...Ch. 16.9 - Predict the position of the following equilibrium.Ch. 16.9 - Draw a structural formula for the keto form of...Ch. 16.10 - Prob. 16.11PCh. 16.11 - What aldehyde or ketone gives these alcohols upon...Ch. 16.11 - Prob. 16.13PCh. 16 - Prob. 16.14PCh. 16 - Prob. 16.15PCh. 16 - The infrared spectrum of compound A, C6H12O, shows...Ch. 16 - Following are 1H-NMR spectra for compounds B...Ch. 16 - Draw structural formulas for the product formed by...Ch. 16 - Suggest a synthesis for the following alcohols...Ch. 16 - Show how to synthesize the following alcohol using...Ch. 16 - 1-Phenyl-2-butanol is used in perfumery. Show how...Ch. 16 - Prob. 16.22PCh. 16 - Draw structural formulas for (1) the...Ch. 16 - Show how to bring about the following conversions...Ch. 16 - Prob. 16.25PCh. 16 - Wittig reactions with the following -chloroethers...Ch. 16 - Prob. 16.27PCh. 16 - Prob. 16.28PCh. 16 - 5-Hydroxyhexanal forms a six-membered cyclic...Ch. 16 - Prob. 16.30PCh. 16 - Prob. 16.31PCh. 16 - Propose a mechanism to account for the formation...Ch. 16 - Prob. 16.33PCh. 16 - Prob. 16.34PCh. 16 - Show how to bring about the following conversion.Ch. 16 - A primary or secondary alcohol can be protected by...Ch. 16 - Prob. 16.37PCh. 16 - Prob. 16.38PCh. 16 - Prob. 16.39PCh. 16 - Prob. 16.40PCh. 16 - The following molecule belongs to a class of...Ch. 16 - When cis-2-decalone is dissolved in ether...Ch. 16 - Prob. 16.43PCh. 16 - Prob. 16.44PCh. 16 - The following bicyclic ketone has two -carbons and...Ch. 16 - Propose a mechanism for this reaction.Ch. 16 - The base-promoted rearrangement of an -haloketone...Ch. 16 - If the Favorskii rearrangement of...Ch. 16 - (R)-Pulegone, readily available from pennyroyal...Ch. 16 - (R)-Pulegone is converted to (R)-citronellic acid...Ch. 16 - Starting with cyclohexanone, show how to prepare...Ch. 16 - Show how to convert cyclopentanone to these...Ch. 16 - Prob. 16.53PCh. 16 - Prob. 16.54PCh. 16 - Prob. 16.55PCh. 16 - Following is the structural formula of Surfynol, a...Ch. 16 - Prob. 16.57PCh. 16 - Propose a mechanism for this isomerization.Ch. 16 - Starting with acetylene and 1-bromobutane as the...Ch. 16 - Prob. 16.60PCh. 16 - Prob. 16.61PCh. 16 - Prob. 16.62PCh. 16 - Prob. 16.63PCh. 16 - Prob. 16.64PCh. 16 - All rearrangements we have discussed so far have...Ch. 16 - In dilute aqueous base, (R)-glyceraldehyde is...Ch. 16 - Treatment of -D-glucose with methanol in the...Ch. 16 - Treating a Grignard reagent with carbon dioxide...Ch. 16 - Prob. 16.69PCh. 16 - Prob. 16.70PCh. 16 - Prob. 16.71PCh. 16 - Prob. 16.72PCh. 16 - Write the products of the following sequences of...Ch. 16 - Using your reaction roadmaps as a guide, show how...Ch. 16 - Using your reaction roadmaps as a guide, show how...Ch. 16 - Using your reaction roadmaps as a guide, show how...Ch. 16 - Using your reaction roadmaps as a guide, show how...Ch. 16 - Prob. 16.78PCh. 16 - Prob. 16.79PCh. 16 - Prob. 16.80PCh. 16 - Prob. 16.81P
Additional Science Textbook Solutions
Find more solutions based on key concepts
How does the removal of hydrogen atoms from nutrient molecules result in a loss of energy from the nutrient mol...
SEELEY'S ANATOMY+PHYSIOLOGY
Separate the list P,F,V,,T,a,m,L,t, and V into intensive properties, extensive properties, and nonproperties.
Fundamentals Of Thermodynamics
Give the IUPAC name for each compound.
Organic Chemistry
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Using reaction free energy to predict equilibrium composition Consider the following equilibrium: 2NOCI (g) 2NO (g) + Cl2 (g) AGº =41. kJ Now suppose a reaction vessel is filled with 4.50 atm of nitrosyl chloride (NOCI) and 6.38 atm of chlorine (C12) at 212. °C. Answer the following questions about this system: ? rise Under these conditions, will the pressure of NOCI tend to rise or fall? x10 fall Is it possible to reverse this tendency by adding NO? In other words, if you said the pressure of NOCI will tend to rise, can that be changed to a tendency to fall by adding NO? Similarly, if you said the pressure of NOCI will tend to fall, can that be changed to a tendency to rise by adding NO? yes no If you said the tendency can be reversed in the second question, calculate the minimum pressure of NO needed to reverse it. Round your answer to 2 significant digits. 0.035 atm ✓ G 00. 18 Ararrow_forwardHighlight each glycosidic bond in the molecule below. Then answer the questions in the table under the drawing area. HO- HO- -0 OH OH HO NG HO- HO- OH OH OH OH NG OHarrow_forward€ + Suppose the molecule in the drawing area below were reacted with H₂ over a platinum catalyst. Edit the molecule to show what would happen to it. That is, turn it into the product of the reaction. Also, write the name of the product molecule under the drawing area. Name: ☐ H C=0 X H- OH HO- H HO- -H CH₂OH ×arrow_forward
- Draw the Haworth projection of the disaccharide made by joining D-glucose and D-mannose with a ẞ(1-4) glycosidic bond. If the disaccharide has more than one anomer, you can draw any of them. Click and drag to start drawing a structure. Xarrow_forwardEpoxides can be opened in aqueous acid or aqueous base to produce diols (molecules with two OH groups). In this question, you'll explore the mechanism of epoxide opening in aqueous acid. 2nd attempt Be sure to show all four bonds at stereocenters using hash and wedge lines. 0 0 Draw curved arrows to show how the epoxide reacts with hydronium ion. 100 +1: 1st attempt Feedback Be sure to show all four bonds at stereocenters using hash and wedge lines. See Periodic Table See Hint H A 5 F F Hr See Periodic Table See Hintarrow_forward03 Question (1 point) For the reaction below, draw both of the major organic products. Be sure to consider stereochemistry. > 1. CH₂CH₂MgBr 2. H₂O 3rd attempt Draw all four bonds at chiral centers. Draw all stereoisomers formed. Draw the structures here. e 130 AN H See Periodic Table See Hint P C Brarrow_forward
- You may wish to address the following issues in your response if they are pertinent to the reaction(s) you propose to employ:1) Chemoselectivity (why this functional group and not another?) 2) Regioselectivity (why here and not there?) 3) Stereoselectivity (why this stereoisomer?) 4) Changes in oxidation state. Please make it in detail and draw it out too in what step what happens. Thank you for helping me!arrow_forward1) Chemoselectivity (why this functional group and not another?) 2) Regioselectivity (why here and not there?) 3) Stereoselectivity (why this stereoisomer?) 4) Changes in oxidation state. Everything in detail and draw out and write it.arrow_forwardCalculating the pH at equivalence of a titration 3/5 Izabella A chemist titrates 120.0 mL of a 0.7191M dimethylamine ((CH3)2NH) solution with 0.5501 M HBr solution at 25 °C. Calculate the pH at equivalence. The pk of dimethylamine is 3.27. Round your answer to 2 decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of HBr solution added. pH = ☐ ✓ 18 Ar Boarrow_forward
- Alcohols can be synthesized using an acid-catalyzed hydration of an alkene. An alkene is combined with aqueous acid (e.. sulfuric acid in water). The reaction mechanism typically involves a carbocation intermediate. > 3rd attempt 3343 10 8 Draw arrows to show the reaction between the alkene and hydronium ion. that 2nd attempt Feedback 1st attempt تعمال Ju See Periodic Table See Hint F D Ju See Periodic Table See Hintarrow_forwardDraw the simplified curved arrow mechanism for the reaction of acetone and CHgLi to give the major product. 4th attempt Π Draw the simplified curved arrow mechanism T 3rd attempt Feedback Ju See Periodic Table See Hint H -H H -I H F See Periodic Table See Hintarrow_forwardSelect the correct reagent to accomplish the first step of this reaction. Then draw a mechanism on the Grignard reagent using curved arrow notation to show how it is converted to the final product. 4th attempt Part 1 (0.5 point) Select the correct reagent to accomplish the first step of this reaction. Choose one: OA Mg in ethanol (EtOH) OB. 2 Li in THF O C. Li in THF D. Mg in THF O E Mg in H2O Part 2 (0.5 point) Br Part 1 Bri Mg CH B CH, 1 Draw intermediate here, but no arrows. © TE See Periodic Table See Hint See Hint ין Harrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning


Enzymes - Effect of cofactors on enzyme; Author: Tutorials Point (India) Ltd;https://www.youtube.com/watch?v=AkAbIwxyUs4;License: Standard YouTube License, CC-BY
Enzyme Catalysis Part-I; Author: NPTEL-NOC IITM;https://www.youtube.com/watch?v=aZE740JWZuQ;License: Standard Youtube License