
(a)
Interpretation:
The given compound’s IUPAC name has to be determined and the relevant stereochemistry should be specified.
Concept introduction:
The functional group in the
Naming Aldehydes:
Aldehydes have at least one hydrogen attached to the carbonyl carbon atom.
The IUPAC naming of aldehydes is obtained by replacing the final "e" on the name of the parent hydrocarbon with "al".
For example:
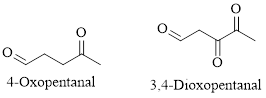
Naming Ketones:
The IUPAC name of a ketones are obtained by replacing the "e" on the end of the parent hydrocarbon with "one".
Only few ketones have common name.
For example:
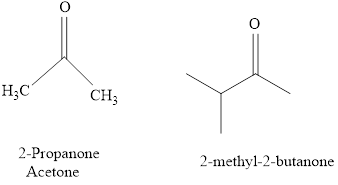
Naming of compounds with two
If a compound has two functional groups, the one with lower priority is indicated by a prefix and another with the higher priority by a suffix.
(a)
Explanation of Solution
The given compound is as follows.
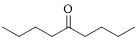
Let’s given numbering to this compound as follows,
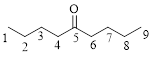
The parent chain contains 9 carbon atoms; in the fifth carbon atom a ketone functional group is attached.
Thus, according to IUPAC this compound can be named as 5-Nonanone.
(b)
Interpretation:
The given compound’s IUPAC name has to be determined and the relevant stereochemistry should be specified.
Concept introduction:
The functional group in the aldehydes and Ketones are carbonyl group.
Naming Aldehydes:
Aldehydes have at least one hydrogen attached to the carbonyl carbon atom.
The IUPAC naming of aldehydes is obtained by replacing the final "e" on the name of the parent hydrocarbon with "al".
For example:
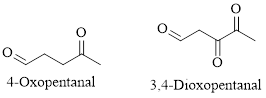
Naming Ketones:
The IUPAC name of a ketones are obtained by replacing the "e" on the end of the parent hydrocarbon with "one".
Only few ketones have common name.
For example:

Naming of compounds with two functional groups,
If a compound has two functional groups, the one with lower priority is indicated by a prefix and another with the higher priority by a suffix.
R and S nomenclature: it is used to assign the molecule using CIP (Cahn-Ingold-Prelog) rules.
The CIP rules are as follows:
Select the chiral carbon and assign the numbers according to the decreasing
If the numbering follows clockwise direction then the molecule is termed as R and if it follows anti-clockwise direction then molecule is termed as S.
(b)
Explanation of Solution
The given compound is as follows.
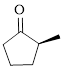
Let’s write give the numbering to this compound.
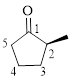
This parent ring has five carbon atoms; a methyl group was attached to the second carbon atom. Therefore, according to the IUPAC rules, the compound can be named as
Here, this compound has a chiral center (it is highlighted as *); its configuration can be specified as follows,
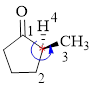
The numbering follows an anti-clock wise direction and so molecule is in as S configuration.
Thus, the compound name can be written as
(c)
Interpretation:
The given compound’s IUPAC name has to be determined and the relevant stereochemistry should be specified.
Concept introduction:
The functional group in the aldehydes and Ketones are carbonyl group.
Naming Aldehydes:
Aldehydes have at least one hydrogen attached to the carbonyl carbon atom.
The IUPAC naming of aldehydes is obtained by replacing the final "e" on the name of the parent hydrocarbon with "al".
For example:
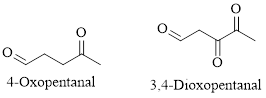
Naming Ketones:
The IUPAC name of a ketones are obtained by replacing the "e" on the end of the parent hydrocarbon with "one".
Only few ketones have common name.
For example:

Naming of compounds with two functional groups;
If a compound has two functional groups, the one with lower priority is indicated by a prefix and another with the higher priority by a suffix.
(c)
Explanation of Solution
The given compound is as follows.
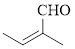
Let’s give the numbering to the given compound.
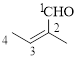
The parent hydrocarbon chain has two functional groups and they are
(d)
Interpretation:
The given compound’s IUPAC name has to be determined and the relevant stereochemistry should be specified.
Concept introduction:
The functional group in the aldehydes and Ketones are carbonyl group.
Naming Aldehydes:
Aldehydes have at least one hydrogen attached to the carbonyl carbon atom.
The IUPAC naming of aldehydes is obtained by replacing the final "e" on the name of the parent hydrocarbon with "al".
For example:
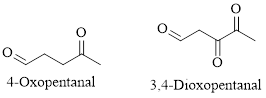
Naming Ketones:
The IUPAC name of a ketones are obtained by replacing the "e" on the end of the parent hydrocarbon with "one".
Only few ketones have common name.
For example:

Naming of compounds with two functional groups;
If a compound has two functional groups, the one with lower priority is indicated by a prefix and another with the higher priority by a suffix.
R and S nomenclature: it is used to assign the molecule using CIP (Cahn-Ingold-Prelog) rules.
The CIP rules are as follows:
Select the chiral carbon and assign the numbers according to the decreasing atomic mass of atoms attached to it.
If the numbering follows clockwise direction then the molecule is termed as R and if it follows anti-clockwise direction then molecule is termed as S.
(d)
Explanation of Solution
The given compound is as follows.
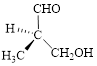
Let’s write give the numbering to this compound.
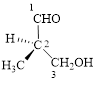
This parent chain has three carbon atoms; a methyl group was attached to the second carbon atom and hydroxyl group was attached to the third carbon atom. Therefore, according to the IUPAC rules, the compound can be named as 3-Hydroxy-2-methyl-propanal.
Here, this compound has a chiral center (it is highlighted as *); its configuration can be specified as follows,
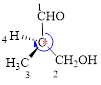
The numbering of substituents on the chiral center follows clock wise direction and so molecule is in R configuration.
Thus, the compound name can be written as (R)-3-Hydroxy-2-methyl-propanal.
(e)
Interpretation:
The given compound’s IUPAC name has to be determined and the relevant stereochemistry should be specified.
Concept introduction:
The functional group in the aldehydes and Ketones are carbonyl group.
Naming Aldehydes:
Aldehydes have at least one hydrogen attached to the carbonyl carbon atom.
The IUPAC naming of aldehydes is obtained by replacing the final "e" on the name of the parent hydrocarbon with "al".
For example:
Naming Ketones:
The IUPAC name of a ketones are obtained by replacing the "e" on the end of the parent hydrocarbon with "one".
Only few ketones have common name.
For example:

Naming of compounds with two functional groups;
If a compound has two functional groups, the one with lower priority is indicated by a prefix and another with the higher priority by a suffix.
(e)
Explanation of Solution
The given compound is as follows.
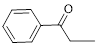
Let’s give the numbering to the given compound.
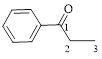
A phenyl ring is attached to the first carbon atom in the three membered parent carbon chains. According to IUPAC the aldehyde group has higher priority. Thus the compound can be named as 1-phenyl-1-propanone.
(f)
Interpretation:
The given compound’s IUPAC name has to be determined and the relevant stereochemistry should be specified.
Concept introduction:
The functional group in the aldehydes and Ketones are carbonyl group.
Naming Aldehydes:
Aldehydes have at least one hydrogen attached to the carbonyl carbon atom.
The IUPAC naming of aldehydes is obtained by replacing the final "e" on the name of the parent hydrocarbon with "al".
For example:
Naming Ketones:
The IUPAC name of a ketones are obtained by replacing the "e" on the end of the parent hydrocarbon with "one".
Only few ketones have common name.
For example:
Naming of compounds with two functional groups;
If a compound has two functional groups, the one with lower priority is indicated by a prefix and another with the higher priority by a suffix.
R and S nomenclature: it is used to assign the molecule using CIP (Cahn-Ingold-Prelog) rules.
The CIP rules are as follows:
Select the chiral carbon and assign the numbers according to the decreasing atomic mass of atoms attached to it.
If the numbering follows clockwise direction then the molecule is termed as R and if it follows anti-clockwise direction then molecule is termed as S.
(f)
Explanation of Solution
The given compound is as follows.
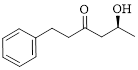
Let’s write give the numbering to this compound.
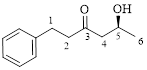
This parent ring has six carbon atoms; a hydroxyl (–OH) and a phenyl ring were attached to the first and fifth carbon atoms in the parent chain respectively. Therefore, according to the IUPAC rules, the compound can be named as 5-Hydroxy-1-phenyl-3-hexanone.
Here, this compound has a chiral center (it is highlighted as *); its configuration can be specified as follows,
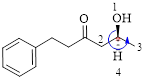
The numbering follows an anti-clock wise direction and so molecule is in as S configuration.
Thus, the compound name can be written as (S)-5-Hydroxy-1-phenyl-3-hexanone.
(g)
Interpretation:
The given compound’s IUPAC name has to be determined and the relevant stereochemistry should be specified.
Concept introduction:
The functional group in the aldehydes and Ketones are carbonyl group.
Naming Aldehydes:
Aldehydes have at least one hydrogen attached to the carbonyl carbon atom.
The IUPAC naming of aldehydes is obtained by replacing the final "e" on the name of the parent hydrocarbon with "al".
For example:
Naming Ketones:
The IUPAC name of a ketones are obtained by replacing the "e" on the end of the parent hydrocarbon with "one".
Only few ketones have common name.
For example:

Naming of compounds with two functional groups;
If a compound has two functional groups, the one with lower priority is indicated by a prefix and another with the higher priority by a suffix.
(g)
Explanation of Solution
The given compound is as follows.
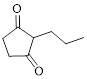
Let’s give the numbering to the given compound.
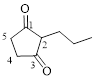
The parent hydrocarbon ring has five carbon atoms. A propyl group was attached to the second carbon atom in the ring and two ketone groups were present in first and third carbon atom respectively. Thus the compound can be named as
(h)
Interpretation:
The given compound’s IUPAC name has to be determined and the relevant stereochemistry should be specified.
Concept introduction:
The functional group in the aldehydes and Ketones are carbonyl group.
Naming Aldehydes:
Aldehydes have at least one hydrogen attached to the carbonyl carbon atom.
The IUPAC naming of aldehydes is obtained by replacing the final "e" on the name of the parent hydrocarbon with "al".
For example:
Naming Ketones:
The IUPAC name of a ketones are obtained by replacing the "e" on the end of the parent hydrocarbon with "one".
Only few ketones have common name.
For example:

Naming of compounds with two functional groups;
If a compound has two functional groups, the one with lower priority is indicated by a prefix and another with the higher priority by a suffix.
(h)
Explanation of Solution
The given compound is as follows.
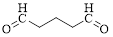
Let’s give the numbering to the given compound.
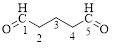
The parent hydrocarbon chain has five carbon atoms with two aldehydes on both ends. Thus according to IUPAC the compound can be named as pentanedial.
(i)
Interpretation:
The given compound’s IUPAC name has to be determined and the relevant stereochemistry should be specified.
Concept introduction:
The functional group in the aldehydes and Ketones are carbonyl group.
Naming Aldehydes:
Aldehydes have at least one hydrogen attached to the carbonyl carbon atom.
The IUPAC naming of aldehydes is obtained by replacing the final "e" on the name of the parent hydrocarbon with "al".
For example:
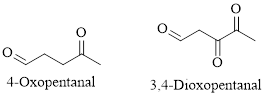
Naming Ketones:
The IUPAC name of a ketones are obtained by replacing the "e" on the end of the parent hydrocarbon with "one".
Only few ketones have common name.
For example:

Naming of compounds with two functional groups;
If a compound has two functional groups, the one with lower priority is indicated by a prefix and another with the higher priority by a suffix.
(i)
Explanation of Solution
The given compound is as follows.
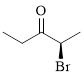
Let’s give the numbering to the given compound.
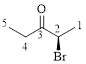
The parent hydrocarbon chain has two functional groups and they are bromine and ketone. According to IUPAC the bromine has higher priority. Thus the compound can be named as 2-bromo-3-pentanone.
Here, this compound has a chiral center (it is highlighted as *); its configuration can be specified as follows,
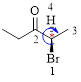
The numbering follows a clock wise direction and so molecule is in R configuration.
Thus, the compound name can be written as (R)-2-bromo-3-pentanone.
Want to see more full solutions like this?
Chapter 16 Solutions
Organic Chemistry
- Identify and provide an explanation of the operational principles behind a Atomic Absorption Spectrometer (AAS). List the steps involved.arrow_forwardInstructions: Complete the questions in the space provided. Show all your work 1. You are trying to determine the rate law expression for a reaction that you are completing at 25°C. You measure the initial reaction rate and the starting concentrations of the reactions for 4 trials. BrO³¯ (aq) + 5Br¯ (aq) + 6H* (aq) → 3Br₂ (l) + 3H2O (l) Initial rate Trial [BrO3] [H*] [Br] (mol/L) (mol/L) | (mol/L) (mol/L.s) 1 0.10 0.10 0.10 8.0 2 0.20 0.10 0.10 16 3 0.10 0.20 0.10 16 4 0.10 0.10 0.20 32 a. Based on the above data what is the rate law expression? b. Solve for the value of k (make sure to include proper units) 2. The proposed reaction mechanism is as follows: i. ii. BrО¸¯ (aq) + H+ (aq) → HBrO3 (aq) HBrO³ (aq) + H* (aq) → H₂BrO3* (aq) iii. H₂BrO³* (aq) + Br¯ (aq) → Br₂O₂ (aq) + H2O (l) [Fast] [Medium] [Slow] iv. Br₂O₂ (aq) + 4H*(aq) + 4Br(aq) → 3Br₂ (l) + H2O (l) [Fast] Evaluate the validity of this proposed reaction. Justify your answer.arrow_forwardе. Д CH3 D*, D20arrow_forward
- H3C. H3C CH 3 CH 3 CH3 1. LDA 2. PhSeCl 3. H2O2arrow_forwardPlease predict the products for each of the following reactions: 1.03 2. H₂O NaNH, 1. n-BuLi 2. Mel A H₂ 10 9 0 H2SO4, H₂O HgSO4 Pd or Pt (catalyst) B 9 2 n-BuLi ♡ D2 (deuterium) Lindlar's Catalyst 1. NaNH2 2. EtBr Na, ND3 (deuterium) 2. H₂O2, NaOH 1. (Sia)2BH с Darrow_forwardin the scope of ontario SCH4U grade 12 course, please show ALL workarrow_forward
- Is the chemical reaction CuCl42-(green) + 4H2O <==> Cu(H2O)42+(blue) + 4Cl- exothermic or endothermic?arrow_forwardIf we react tetraethoxypropane with hydrazine, what is the product obtained (explain its formula). State the reason why the corresponding dialdehyde is not used.arrow_forwarddrawing, no aiarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
