
Chemistry: An Introduction to General, Organic, and Biological Chemistry (12th Edition) - Standalone book
12th Edition
ISBN: 9780321908445
Author: Karen C. Timberlake
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16.6, Problem 16.46QAP
The following graph shows the activity versus pH curves for pepsin, sucrase, and trypsin. Estimate the optimum pH for each.
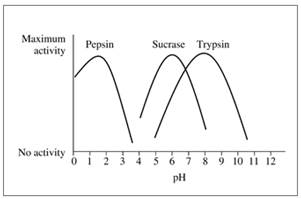
16.46 Refer to the graph in problem 16.45 to determine if the reaction rate in each condition will be at the optimum rate or not.
- Trypsin, pH 5.0
- Sucrase, pH 5.0
- Pepsin, pH 4.0
- Trypsin, pH 8.0
- Pepsin, pH 2.0
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
None
3. Consider the compounds below and determine if they are aromatic, antiaromatic, or
non-aromatic. In case of aromatic or anti-aromatic, please indicate number of I
electrons in the respective systems. (Hint: 1. Not all lone pair electrons were explicitly
drawn and you should be able to tell that the bonding electrons and lone pair electrons
should reside in which hybridized atomic orbital 2. You should consider ring strain-
flexibility and steric repulsion that facilitates adoption of aromaticity or avoidance of anti-
aromaticity)
H H
N
N:
NH2
N
Aromaticity
(Circle)
Aromatic Aromatic Aromatic Aromatic Aromatic
Antiaromatic Antiaromatic Antiaromatic Antiaromatic Antiaromatic
nonaromatic nonaromatic nonaromatic nonaromatic nonaromatic
aromatic TT
electrons
Me
H
Me
Aromaticity
(Circle)
Aromatic Aromatic Aromatic
Aromatic Aromatic
Antiaromatic Antiaromatic Antiaromatic Antiaromatic Antiaromatic
nonaromatic nonaromatic nonaromatic nonaromatic nonaromatic
aromatic πT
electrons
H
HH…
A chemistry graduate student is studying the rate of this reaction:
2 HI (g) →H2(g) +12(g)
She fills a reaction vessel with HI and measures its concentration as the reaction proceeds:
time
(minutes)
[IH]
0
0.800M
1.0
0.301 M
2.0
0.185 M
3.0
0.134M
4.0
0.105 M
Use this data to answer the following questions.
Write the rate law for this reaction.
rate
= 0
Calculate the value of the rate constant k.
k =
Round your answer to 2 significant digits. Also be
sure your answer has the correct unit symbol.
Chapter 16 Solutions
Chemistry: An Introduction to General, Organic, and Biological Chemistry (12th Edition) - Standalone book
Ch. 16.1 - Classify each of the following proteins according...Ch. 16.1 - Prob. 16.2QAPCh. 16.1 - Prob. 16.3QAPCh. 16.1 - Prob. 16.4QAPCh. 16.1 - Draw the structure for each of the following amino...Ch. 16.1 - Draw the structure for each of the following amino...Ch. 16.1 - Draw the strcture for each of the following amino...Ch. 16.1 - Prob. 16.8QAPCh. 16.1 - Prob. 16.9QAPCh. 16.1 - Prob. 16.10QAP
Ch. 16.2 - Prob. 16.11QAPCh. 16.2 - Prob. 16.12QAPCh. 16.2 - Prob. 16.13QAPCh. 16.2 - Prob. 16.14QAPCh. 16.2 - Prob. 16.15QAPCh. 16.2 - Prob. 16.16QAPCh. 16.3 - Prob. 16.17QAPCh. 16.3 - Prob. 16.18QAPCh. 16.3 - Prob. 16.19QAPCh. 16.3 - Prob. 16.20QAPCh. 16.4 - Prob. 16.21QAPCh. 16.4 - Prob. 16.22QAPCh. 16.4 - Prob. 16.23QAPCh. 16.4 - Prob. 16.24QAPCh. 16.4 - What type of interaction would you expect between...Ch. 16.4 - What type of interaction would you expect between...Ch. 16.4 - Prob. 16.27QAPCh. 16.4 - Prob. 16.28QAPCh. 16.4 - Prob. 16.29QAPCh. 16.4 - Prob. 16.30QAPCh. 16.4 - Prob. 16.31QAPCh. 16.4 - Indicate the changes in secondary and tertiary...Ch. 16.5 - Why do chemical reactions in the body require...Ch. 16.5 - Prob. 16.34QAPCh. 16.5 - Prob. 16.35QAPCh. 16.5 - Prob. 16.36QAPCh. 16.5 - Prob. 16.37QAPCh. 16.5 - Prob. 16.38QAPCh. 16.5 - Prob. 16.39QAPCh. 16.5 - 16.36 Match the terms (1) active site, (2)...Ch. 16.5 - Prob. 16.51QAPCh. 16.5 - Prob. 16.52QAPCh. 16.5 - Prob. 16.43QAPCh. 16.5 - Prob. 16.44QAPCh. 16.5 - For problems 16.39 to 16.42, see Chemistry Link to...Ch. 16.5 - Prob. 16.46QAPCh. 16.6 - Trypsin, a peptidase that hydrolyzes polypeptides,...Ch. 16.6 - pepsin, a peptidase that hydrolyzes proteins,...Ch. 16.6 - The following graph shows the activity versus pH...Ch. 16.6 - The following graph shows the activity versus pH...Ch. 16.6 - Prob. 16.47QAPCh. 16.6 - Prob. 16.48QAPCh. 16.6 - Prob. 16.49QAPCh. 16.6 - Prob. 16.50QAPCh. 16.6 - What is the chemical formula for hydroxyurea?Ch. 16.6 - What is the molar mass of hydroxyurea?Ch. 16.6 - Prob. 16.53QAPCh. 16.6 - Prob. 16.54QAPCh. 16 - Prob. 16.55UTCCh. 16 - Prob. 16.56UTCCh. 16 - Prob. 16.57UTCCh. 16 - Prob. 16.58UTCCh. 16 - Prob. 16.59UTCCh. 16 - Prob. 16.60UTCCh. 16 - 16.59 Identify the amino acids and type of...Ch. 16 - What type of interaction would you expect between...Ch. 16 - Draw the condensed structural formula for...Ch. 16 - Draw the condensed structural formula for...Ch. 16 - Seed and vegetables are often deficient in one or...Ch. 16 - 16.64 Seeds and vegetables are often deficient in...Ch. 16 - Prob. 16.67AQAPCh. 16 - Prob. 16.68AQAPCh. 16 - Prob. 16.69AQAPCh. 16 - Prob. 16.70AQAPCh. 16 - Prob. 16.71AQAPCh. 16 - Why do enzymes function only under mild...Ch. 16 - Prob. 16.73AQAPCh. 16 - Prob. 16.74AQAPCh. 16 - Prob. 16.75AQAPCh. 16 - Prob. 16.76AQAPCh. 16 - Prob. 16.77AQAPCh. 16 - Prob. 16.78AQAPCh. 16 - Prob. 16.79AQAPCh. 16 - Prob. 16.80AQAPCh. 16 - If a blood test indicates a high level of LDH and...Ch. 16 - Prob. 16.82AQAPCh. 16 - Prob. 16.83CQCh. 16 - Prob. 16.84CQCh. 16 - Prob. 16.85CQCh. 16 - Prob. 16.86CQ
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1. For the four structures provided, Please answer the following questions in the table below. a. Please draw π molecular orbital diagram (use the polygon-and-circle method if appropriate) and fill electrons in each molecular orbital b. Please indicate the number of π electrons c. Please indicate if each molecule provided is anti-aromatic, aromatic, or non- aromatic TT MO diagram Number of π e- Aromaticity Evaluation (X choose one) Non-aromatic Aromatic Anti-aromatic || ||| + IVarrow_forward1.3 grams of pottasium iodide is placed in 100 mL of o.11 mol/L lead nitrate solution. At room temperature, lead iodide has a Ksp of 4.4x10^-9. How many moles of precipitate will form?arrow_forwardQ3: Circle the molecules that are optically active: ДДДДarrow_forward
- 6. How many peaks would be observed for each of the circled protons in the compounds below? 8 pts CH3 CH3 ΤΙ A. H3C-C-C-CH3 I (₁₁ +1)= 7 H CI B. H3C-C-CI H (3+1)=4 H LIH)=2 C. (CH3CH2-C-OH H D. CH3arrow_forwardNonearrow_forwardQ1: Draw the most stable and the least stable Newman projections about the C2-C3 bond for each of the following isomers (A-C). Are the barriers to rotation identical for enantiomers A and B? How about the diastereomers (A versus C or B versus C)? H Br H Br (S) CH3 (R) CH3 H3C (S) H3C H Br Br H A C enantiomers H Br H Br (R) CH3 H3C (R) (S) CH3 H3C H Br Br H B D identicalarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co
DIGESTER-35 | VITAMINS AND THEIR RELATED COENZYMES| GPAT | NIPER | PHARMACIST| DI; Author: GPAT DISCUSSION CENTER;https://www.youtube.com/watch?v=CGrdNYmho0s;License: Standard YouTube License, CC-BY