
Chemistry: An Introduction to General, Organic, and Biological Chemistry (12th Edition) - Standalone book
12th Edition
ISBN: 9780321908445
Author: Karen C. Timberlake
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Question
Chapter 16.2, Problem 16.15QAP
Interpretation Introduction
To determine: The pH of
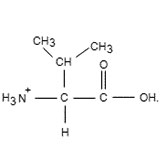
Interpretation Introduction
To determine: The pH of ionized form.
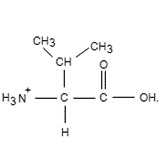
Interpretation Introduction
To determine: The pH of ionized form
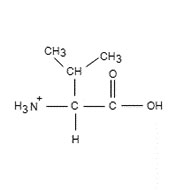
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
None
None
4. Draw and label all possible isomers for [M(py)3(DMSO)2(CI)] (py = pyridine, DMSO
dimethylsulfoxide).
Chapter 16 Solutions
Chemistry: An Introduction to General, Organic, and Biological Chemistry (12th Edition) - Standalone book
Ch. 16.1 - Classify each of the following proteins according...Ch. 16.1 - Prob. 16.2QAPCh. 16.1 - Prob. 16.3QAPCh. 16.1 - Prob. 16.4QAPCh. 16.1 - Draw the structure for each of the following amino...Ch. 16.1 - Draw the structure for each of the following amino...Ch. 16.1 - Draw the strcture for each of the following amino...Ch. 16.1 - Prob. 16.8QAPCh. 16.1 - Prob. 16.9QAPCh. 16.1 - Prob. 16.10QAP
Ch. 16.2 - Prob. 16.11QAPCh. 16.2 - Prob. 16.12QAPCh. 16.2 - Prob. 16.13QAPCh. 16.2 - Prob. 16.14QAPCh. 16.2 - Prob. 16.15QAPCh. 16.2 - Prob. 16.16QAPCh. 16.3 - Prob. 16.17QAPCh. 16.3 - Prob. 16.18QAPCh. 16.3 - Prob. 16.19QAPCh. 16.3 - Prob. 16.20QAPCh. 16.4 - Prob. 16.21QAPCh. 16.4 - Prob. 16.22QAPCh. 16.4 - Prob. 16.23QAPCh. 16.4 - Prob. 16.24QAPCh. 16.4 - What type of interaction would you expect between...Ch. 16.4 - What type of interaction would you expect between...Ch. 16.4 - Prob. 16.27QAPCh. 16.4 - Prob. 16.28QAPCh. 16.4 - Prob. 16.29QAPCh. 16.4 - Prob. 16.30QAPCh. 16.4 - Prob. 16.31QAPCh. 16.4 - Indicate the changes in secondary and tertiary...Ch. 16.5 - Why do chemical reactions in the body require...Ch. 16.5 - Prob. 16.34QAPCh. 16.5 - Prob. 16.35QAPCh. 16.5 - Prob. 16.36QAPCh. 16.5 - Prob. 16.37QAPCh. 16.5 - Prob. 16.38QAPCh. 16.5 - Prob. 16.39QAPCh. 16.5 - 16.36 Match the terms (1) active site, (2)...Ch. 16.5 - Prob. 16.51QAPCh. 16.5 - Prob. 16.52QAPCh. 16.5 - Prob. 16.43QAPCh. 16.5 - Prob. 16.44QAPCh. 16.5 - For problems 16.39 to 16.42, see Chemistry Link to...Ch. 16.5 - Prob. 16.46QAPCh. 16.6 - Trypsin, a peptidase that hydrolyzes polypeptides,...Ch. 16.6 - pepsin, a peptidase that hydrolyzes proteins,...Ch. 16.6 - The following graph shows the activity versus pH...Ch. 16.6 - The following graph shows the activity versus pH...Ch. 16.6 - Prob. 16.47QAPCh. 16.6 - Prob. 16.48QAPCh. 16.6 - Prob. 16.49QAPCh. 16.6 - Prob. 16.50QAPCh. 16.6 - What is the chemical formula for hydroxyurea?Ch. 16.6 - What is the molar mass of hydroxyurea?Ch. 16.6 - Prob. 16.53QAPCh. 16.6 - Prob. 16.54QAPCh. 16 - Prob. 16.55UTCCh. 16 - Prob. 16.56UTCCh. 16 - Prob. 16.57UTCCh. 16 - Prob. 16.58UTCCh. 16 - Prob. 16.59UTCCh. 16 - Prob. 16.60UTCCh. 16 - 16.59 Identify the amino acids and type of...Ch. 16 - What type of interaction would you expect between...Ch. 16 - Draw the condensed structural formula for...Ch. 16 - Draw the condensed structural formula for...Ch. 16 - Seed and vegetables are often deficient in one or...Ch. 16 - 16.64 Seeds and vegetables are often deficient in...Ch. 16 - Prob. 16.67AQAPCh. 16 - Prob. 16.68AQAPCh. 16 - Prob. 16.69AQAPCh. 16 - Prob. 16.70AQAPCh. 16 - Prob. 16.71AQAPCh. 16 - Why do enzymes function only under mild...Ch. 16 - Prob. 16.73AQAPCh. 16 - Prob. 16.74AQAPCh. 16 - Prob. 16.75AQAPCh. 16 - Prob. 16.76AQAPCh. 16 - Prob. 16.77AQAPCh. 16 - Prob. 16.78AQAPCh. 16 - Prob. 16.79AQAPCh. 16 - Prob. 16.80AQAPCh. 16 - If a blood test indicates a high level of LDH and...Ch. 16 - Prob. 16.82AQAPCh. 16 - Prob. 16.83CQCh. 16 - Prob. 16.84CQCh. 16 - Prob. 16.85CQCh. 16 - Prob. 16.86CQ
Knowledge Booster
Similar questions
- The emission data in cps displayed in Table 1 is reported to two decimal places by the chemist. However, the instrument output is shown in Table 2. Table 2. Iron emission from ICP-AES Sample Blank Standard Emission, cps 579.503252562 9308340.13122 Unknown Sample 343.232365741 Did the chemist make the correct choice in how they choose to display the data up in Table 1? Choose the best explanation from the choices below. No. Since the instrument calculates 12 digits for all values, they should all be kept and not truncated. Doing so would eliminate significant information. No. Since the instrument calculates 5 decimal places for the standard, all of the values should be limited to the same number. The other decimal places are not significant for the blank and unknown sample. Yes. The way Saman made the standards was limited by the 250-mL volumetric flask. This glassware can report values to 2 decimal places, and this establishes our number of significant figures. Yes. Instrumental data…arrow_forwardSteps and explanation pleasearrow_forwardSteps and explanation to undertand concepts.arrow_forward
- Nonearrow_forward7. Draw a curved arrow mechanism for the following reaction. HO cat. HCI OH in dioxane with 4A molecular sievesarrow_forwardTry: Convert the given 3D perspective structure to Newman projection about C2 - C3 bond (C2 carbon in the front). Also, show Newman projection of other possible staggered conformers and circle the most stable conformation. Use the template shown. F H3C Br Harrow_forward
- Nonearrow_forward16. Consider the probability distribution p(x) = ax", 0 ≤ x ≤ 1 for a positive integer n. A. Derive an expression for the constant a, to normalize p(x). B. Compute the average (x) as a function of n. C. Compute σ2 = (x²) - (x)², the variance of x, as a function of n.arrow_forward451. Use the diffusion model from lecture that showed the likelihood of mixing occurring in a lattice model with eight lattice sites: Case Left Right A B C Permeable Barrier → and show that with 2V lattice sites on each side of the permeable barrier and a total of 2V white particles and 2V black particles, that perfect de-mixing (all one color on each side of the barrier) becomes increasingly unlikely as V increases.arrow_forward
- 46. Consider an ideal gas that occupies 2.50 dm³ at a pressure of 3.00 bar. If the gas is compressed isothermally at a constant external pressure so that the final volume is 0.500 dm³, calculate the smallest value Rest can have. Calculate the work involved using this value of Rext.arrow_forwardNonearrow_forward2010. Suppose that a 10 kg mass of iron at 20 C is dropped from a heigh of 100 meters. What is the kinetics energy of the mass just before it hits the ground, assuming no air resistance? What is its speed? What would be the final temperature of the mass if all the kinetic energy at impact is transformed into internal energy? The molar heat capacity of iron is Cpp = 25.1J mol-¹ K-1 and the gravitational acceleration constant is 9.8 m s¯² |arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY