
(a)
Interpretation:
To identify the
Concept introduction:
The synthesis of primary amine is done by using phthalimide and primary alkyl halide in presence of hydroxide base. The important point for this reaction is the phthalimide group has only one hydrogen atom which is attached to nitrogen and can be replaced by alkyl group. Therefore, only one alkyl group can be substitued to the nitrogen atom and so only primary amine will form as the product. The general reaction equation is written as,
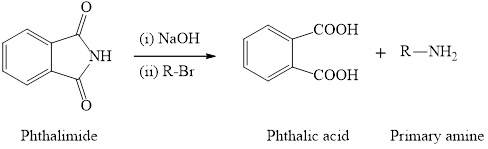
(b)
Interpretation: To identify the alkyl halide used for the preparation of following amines by Gabriel synthesis.
Concept introduction: The synthesis of primary amine is done by using phthalimide and primary alkyl halide in presence of hydroxide base. The important point for this reaction is the phthalimide group has only one hydrogen atom which is attached to nitrogen and can be replaced by alkyl group. Therefore, only one alkyl group can be substitued to the nitrogen atom and so only primary amine will form as the product. The general reaction equation is written as,
(c)
Interpretation:
To identify the alkyl halide used for the preparation of following amines by Gabriel synthesis.
Concept introduction:
The synthesis of primary amine is done by using phthalimide and primary alkyl halide in presence of hydroxide base. The important point for this reaction is the phthalimide group has only one hydrogen atom which is attached to nitrogen and can be replaced by alkyl group. Therefore, only one alkyl group can be substitued to the nitrogen atom and so only primary amine will form as the product. The general reaction equation is written as,
(d)
Interpretation:
To identify the alkyl halide used for the preparation of following amines by Gabriel synthesis.
Concept introduction: The synthesis of primary amine is done by using phthalimide and primary alkyl halide in presence of hydroxide base. The important point for this reaction is the phthalimide group has only one hydrogen atom which is attached to nitrogen and can be replaced by alkyl group. Therefore, only one alkyl group can be substitued to the nitrogen atom and so only primary amine will form as the product. The general reaction equation is written as,
Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
Organic Chemistry
- Identify any polar covalent bonds in epichlorohydrin with S+ and 8- symbols in the appropriate locations. Choose the correct answer below. Η H's+ 6Η Η Η Η Η Ηδ Η Ο Ο HH +Η Η +Η Η Η -8+ CIarrow_forwardH H:O::::H H H HH H::O:D:D:H HH HH H:O:D:D:H .. HH H:O:D:D:H H H Select the correct Lewis dot structure for the following compound: CH3CH2OHarrow_forwardRank the following compounds in order of decreasing boiling point. ннннн -С-С-Н . н-с- ННННН H ΗΤΗ НННН TTTĪ н-с-с-с-с-о-н НННН НН C' Н н-с-с-с-с-н НН || Ш НННН H-C-C-C-C-N-H ННННН IVarrow_forward
- Rank the following compounds in order of decreasing dipole moment. |>||>||| ||>|||>| |>|||>|| |||>||>| O ||>>||| H F H F H c=c || H c=c F F IIIarrow_forwardchoose the description that best describes the geometry for the following charged species ch3-arrow_forwardWhy isn't the ketone in this compound converted to an acetal or hemiacetal by the alcohol and acid?arrow_forward
- What is the approximate bond angle around the nitrogen atom? HNH H Harrow_forwardOH 1. NaOCH2CH3 Q 2. CH3CH2Br (1 equiv) H3O+ Select to Draw 1. NaOCH2 CH3 2. CH3Br (1 equiv) heat Select to Edit Select to Drawarrow_forwardComplete and balance the following half-reaction in acidic solution. Be sure to include the proper phases for all species within the reaction. S₂O₃²⁻(aq) → S₄O₆²⁻(aq)arrow_forward
- Q Select to Edit NH3 (CH3)2CHCI (1 equiv) AICI 3 Select to Draw cat. H2SO4 SO3 (1 equiv) HO SOCl2 pyridine Select to Edit >arrow_forwardComplete and balance the following half-reaction in basic solution. Be sure to include the proper phases for all species within the reaction. Zn(s) → Zn(OH)₄²⁻(aq)arrow_forwardb. ὋΗ CH3CH2OH H2SO4arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning



