
Concept explainers
(a)
Interpretation:
To identify the reagents used to convert methyl propanoate to the following compounds.
Concept introduction:
A reagent is a substance used to convert one chemical compound into another.
The structural formula for methyl propanoate is:
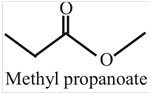
This compound can be converted into different compounds by undergoing a
(b)
Interpretation:
To identify the reagents used to convert methyl propanoate to the following compounds.
Concept introduction:
A reagent is a substance used to convert one chemical compound into another.
The structural formula for methyl propanoate is:

This compound can be converted into different compounds by undergoing a chemical reaction with reagents like water,
(c)
Interpretation:
To identify the reagents used to convert methyl propanoate to the following compounds.
Concept introduction:
A reagent is a substance used to convert one chemical compound into another.
The structural formula for methyl propanoate is:

This compound can be converted into different compounds by undergoing a chemical reaction with reagents like water,
(d)
Interpretation:
To identify the reagents used to convert methyl propanoate to the following compounds.
Concept introduction:
A reagent is a substance used to convert one chemical compound into another.
The structural formula for methyl propanoate is:

This compound can be converted into different compounds by undergoing a chemical reaction with reagents like water,
Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
Organic Chemistry
- 10. Write out the mechanism (intermediate/transition state) for this reaction; indicate stereochemistry in product. H3C CH₂OH CH3 SN1 Harrow_forwardWrite "most" under the member of each trio which is most stable. Write "least under the member of each trio which is least stable. b) Draw a Fischer projection of a pair of enantiomers with three chiral carbons. Which of these two would you expect to be more soluble in water? Why? 1-butanol 1-heptanol Which of these two would you expect to have the higher boiling point? Why? hexyl methyl ether 1-heptanolarrow_forwardWrite "most" under the most acidic compound. Write "least" under the least acidic compound. OH NO₂ OCH3 Br 9. Compound X, C50H84F2, reacts with excess H2/Pd to give a C50H88F2 compound. How many rings are in X? How many double bonds are in X? Show your work.arrow_forward
- 4. State whether these two are: a) the same molecule b) c) d) different compounds that are not isomers constitutional isomers diastereomers e) enantiomers CH3 CH₁₂ H OH HO H H OH HO H CH, CH₂ 5. a) How many stereocenters does this compound have? b) How many stereoisomers are possible for this compound? CH₂ OH CHCHarrow_forwardCalculating the pH at equivalence of a titration A chemist titrates 210.0 mL of a 0.1003 M hydrobromic acid (HBr) solution with 0.7550M KOH solution at 25 °C. Calculate the pH at equivalence. Round your answer to 2 decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of KOH solution added. pH = ] ☑ o0o 18 Ararrow_forwardDo you do chemistry assignmentsarrow_forward
- Using the conditions of spontaneity to deduce the signs of AH and AS Use the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy AS. Note: if you have not been given enough information to decide a sign, select the "unknown" option. reaction observations conclusions A This reaction is always spontaneous, but proceeds slower at temperatures above 120. °C. ΔΗ is (pick one) AS is (pick one) ΔΗ is (pick one) B This reaction is spontaneous except above 117. °C. AS is (pick one) ΔΗ is (pick one) This reaction is slower below 20. °C than C above. AS is |(pick one) ? 18 Ar 1arrow_forwardCalculating the pH at equivalence of a titration Try Again Your answer is incorrect. 0/5 a A chemist titrates 70.0 mL of a 0.7089 M hydrocyanic acid (HCN) solution with 0.4574M KOH solution at 25 °C. Calculate the pH at equivalence. The pK of hydrocyanic acid is 9.21. Round your answer to 2 decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of KOH solution added. pH = 11.43] G 00. 18 Ar B•arrow_forwardBiological Macromolecules Naming and drawing the products of aldose oxidation and reduction aw a Fischer projection of the molecule that would produce L-ribonic acid if it were subjected to mildly oxidizing reaction conditions. Click and drag to start drawing a structure. X AP ‡ 1/5 Naor Explanation Check McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center Accessibilarrow_forward
- ● Biological Macromolecules Identifying the parts of a disaccharide Take a look at this molecule, and then answer the questions in the table below it. CH2OH O H H H OH OH OH H H CH2OH H O OH H OH H H H H OH Is this a reducing sugar? Does this molecule contain a glycosidic bond? If you said this molecule does contain a glycosidic bond, write the symbol describing it. If you said this molecule does contain a glycosidic bond, write the common names (including anomer and enantiomer labels) of the molecules that would be released if that bond were hydrolyzed. If there's more than one molecule, separate each name with a comma. Explanation Check O yes X O no ○ yes O no Uarrow_forwardThe aim of the lab is to measure the sodium content from tomato sauce using the Mohr titration method. There are two groups being: Regular Tomato sauce & Salt Reduced tomato sauce QUESTION: State how you would prepare both Regular & Salt reduced tomato sauce samples for chemical analysis using the Mohr titration methodarrow_forwardUsing the conditions of spontaneity to deduce the signs of AH and AS Use the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy AS. Note: if you have not been given enough information to decide a sign, select the "unknown" option. reaction observations conclusions A The reverse of this reaction is always spontaneous but proceeds faster at temperatures above -48. °C. ΔΗ is (pick one) ✓ AS is (pick one) B This reaction is spontaneous except below 114. °C but proceeds at a slower rate below 135. °C. ΔΗ is (pick one) AS is (pick one) ΔΗ is C This reaction is exothermic and proceeds faster at temperatures above -43. °C. (pick one) AS is (pick one) v Х 5 ? 18 Ararrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER

