
(a)
Interpretation:
To predict the product of acid-catalyzed hydrolysis of given esters.
Concept introduction:
An acid-catalyzed hydrolysis of the ester is a much faster reaction as compared to uncatalyzed hydrolysis of the ester. The addition of acid protonation of the oxygen atom of the carbonyl group and as it is a fact that an oxygen atom with positive charge has more electron withdrawing tendency than neutral atom. The more withdrawal of electron density decreases the electron density from the carbonyl carbon and make it more susceptible for the attack of the nucleophile. The acid catalyzed reaction mechanism is written as,
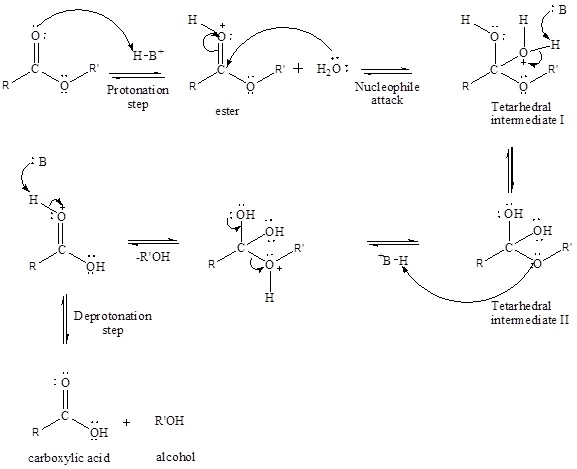
Therefore, products obtained by the acid catalyzed ester hydrolysis are the
(b)
Interpretation:
To predict the product of acid-catalyzed hydrolysis of given esters.
Concept introduction: An acid-catalyzed hydrolysis of the ester is a much faster reaction as compared to uncatalyzed hydrolysis of the ester. The addition of acid protonation of the oxygen atom of the carbonyl group and as it is a fact that an oxygen atom with positive charge has more electron withdrawing tendency than neutral atom. The more withdrawal of electron density decreases the electron density from the carbonyl carbon and make it more susceptible for the attack of the nucleophile. The acid catalyzed reaction mechanism is written as,

Therefore, products obtained by the acid catalyzed ester hydrolysis are the carboxylic acid and an alcohol by which ester was formed.
(c)
Interpretation:
To predict the product of acid-catalyzed hydrolysis of given esters.
Concept introduction:
An acid-catalyzed hydrolysis of the ester is a much faster reaction as compared to uncatalyzed hydrolysis of the ester. The addition of acid protonation of the oxygen atom of the carbonyl group and as it is a fact that an oxygen atom with positive charge has more electron withdrawing tendency than neutral atom. The more withdrawal of electron density decreases the electron density from the carbonyl carbon and make it more susceptible for the attack of the nucleophile. The acid catalyzed reaction mechanism is written as,

Therefore, products obtained by the acid catalyzed ester hydrolysis are the carboxylic acid and an alcohol by which ester was formed.
Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
Organic Chemistry; Modified MasteringChemistry with Pearson eText -- ValuePack Access Card; Study Guide and Student Solutions Manual for Organic Chemistry, Books a la Carte Edition (7th Edition)
- How many chiral centers are there in the following molecule? HO 0 1 ○ 2 ♡ 4 'N'arrow_forwardThe following chemical structure represents a molecule of what molecular formula?arrow_forwardWhich region(s) of the following phospholipid is/are hydrophobic? RO I hydro-water phobic-dislikes = Hydrophobic dislikes water ○ I only Il only I and III only II and IV only O II, III, and IV only III || IVarrow_forward
- Given the following data, determine the order of the reaction with respect to H2. H2(g) + 21Cl(g) → I2(g) + 2HCl(g) Experiment [H2] (torr) [ICI] (torr) Rate (M/s) 1 250 325 0.266 2 250 81 0.0665 3 50 325 0.266arrow_forwardWhich one of the following molecules is chiral? H- NH₂ H3C དང་།་ OH H HO H₂N HO- -H CHO -OH H HO- OH H- -H CH₂OH OHarrow_forwardThe structure of an unsaturated phospholipid is shown below. Which region of the molecule is most hydrophilic ? H₂N-CH₂ H₂C IV CH3 CH3 hydro-water philic-likes = Hydrophilic likes water ○ IV All regions are equally hydrophilic. IIIarrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning




