
(a)
Interpretation:
To suggest two methods one starting with an alcohol and one starting with
Concept introduction:
Esters can be prepared from the reaction of an alcohol with
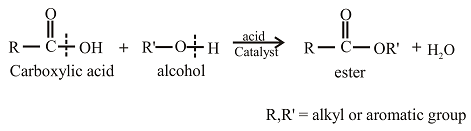
The oxygen atom in the ester is the oxygen atom that is initially present in the alcohol.
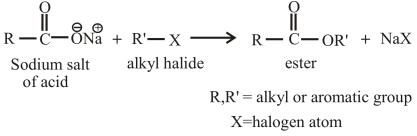
The reaction of an alkyl halide with the sodium salt of carboxylic acid can also be used to prepare esters. The general reaction between an alkyl halide and the sodium salt of carboxylic acid can be given as,
(b)
Interpretation:
To suggest two methods one starting with an alcohol and one starting with alkyl halide for each of the given ester formations.
Concept introduction:
Esters can be prepared from the reaction of an alcohol with carboxylic acid. The general reaction between acid and alcohol can be given as,

The oxygen atom in the ester is the oxygen atom that is initially present in the alcohol.
The reaction of an alkyl halide with the sodium salt of carboxylic acid can also be used to prepare esters. The general reaction between an alkyl halide and the sodium salt of carboxylic acid can be given as,

(c)
Interpretation:
To suggest two methods one starting with an alcohol and one starting with alkyl halide for each of the given ester formations.
Concept introduction:
Esters can be prepared from the reaction of an alcohol with carboxylic acid. The general reaction between acid and alcohol can be given as,

The oxygen atom in the ester is the oxygen atom that is initially present in the alcohol.
The reaction of an alkyl halide with the sodium salt of carboxylic acid can also be used to prepare esters. The general reaction between an alkyl halide and the sodium salt of carboxylic acid can be given as,

(d)
Interpretation:
To suggest two methods one starting with an alcohol and one starting with alkyl halide for each of the given ester formations.
Concept introduction:
Esters can be prepared from the reaction of an alcohol with carboxylic acid. The general reaction between acid and alcohol can be given as,

The oxygen atom in the ester is the oxygen atom that is initially present in the alcohol.
The reaction of an alkyl halide with the sodium salt of carboxylic acid can also be used to prepare esters. The general reaction between an alkyl halide and the sodium salt of carboxylic acid can be given as,

Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
Organic Chemistry; Modified MasteringChemistry with Pearson eText -- ValuePack Access Card; Study Guide and Student Solutions Manual for Organic Chemistry, Books a la Carte Edition (7th Edition)
- How many chiral centers are there in the following molecule? HO 0 1 ○ 2 ♡ 4 'N'arrow_forwardThe following chemical structure represents a molecule of what molecular formula?arrow_forwardWhich region(s) of the following phospholipid is/are hydrophobic? RO I hydro-water phobic-dislikes = Hydrophobic dislikes water ○ I only Il only I and III only II and IV only O II, III, and IV only III || IVarrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning



