
(a)
Interpretation:
The
Concept Introduction:
The addition of one molecule of alcohol to an
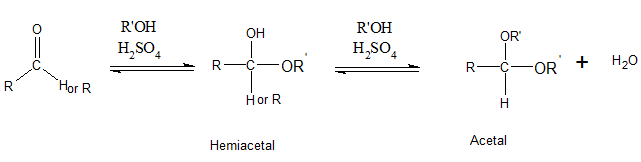
Even though acetals contain C-O-R bond, acetals are not ethers. Acetals have 2 OR groups attached to a single C atom. But an ether has one O atom which is bonded to 2 carbons.
Alcohols are compounds which have OH groups.
(b)
Interpretation:
The functional groups in the following compound should be labeled as an alcohol, ether, acetal or hemiacetal:
Concept Introduction:
The addition of one molecule of alcohol to an aldehyde or ketone results in a hemiacetal. Here, the C=O double bond breaks and single bonds are formed. If the hemiacetal is acyclic, it is not stable. Acyclic hemiacetal reacts with a second alcohol molecule and forms acetal.
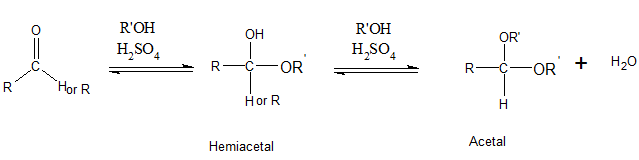
Even though acetals contain C-O-R bond, acetals are not ethers. Acetals have 2 OR groups attached to a single C atom. But an ether has one O atom which is bonded to 2 carbons.
Alcohols are compounds which have OH groups.
Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
CONNECT IA GENERAL ORGANIC&BIO CHEMISTRY
- Draw the product and the mechanism A. excess H*; 人 OH H*; B. C. D. excess OH ✓ OH H*; H₂O 1. LDA 2. H*arrow_forwardIn reactions whose kinetic equation is v = k[A]m, the rate coefficient k is always positive. Is this correct?arrow_forwardIf the concentration of A decreases exponentially with time, what is the rate equation? (A). -d[A] (B). dt d[A] = k[A] e-kt dtarrow_forward
- How many chiral centers are there in the following molecule? HO 0 1 ○ 2 ♡ 4 'N'arrow_forwardThe following chemical structure represents a molecule of what molecular formula?arrow_forwardWhich region(s) of the following phospholipid is/are hydrophobic? RO I hydro-water phobic-dislikes = Hydrophobic dislikes water ○ I only Il only I and III only II and IV only O II, III, and IV only III || IVarrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning


