
Concept explainers
Compounds Y and Z are isomers with the molecular formula
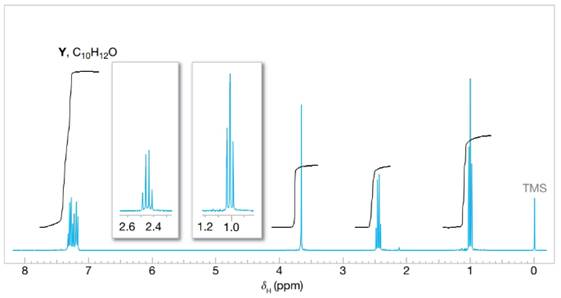
FIGURE 16.4 The
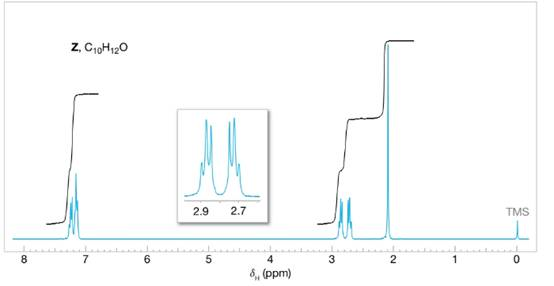
FIGURE 16.5 the
Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
ORGANIC CHEMISTRY-ETEXT REG ACCESS
Additional Science Textbook Solutions
Biochemistry: Concepts and Connections (2nd Edition)
Introductory Chemistry (6th Edition)
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Chemistry (7th Edition)
Human Anatomy & Physiology (2nd Edition)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
- Don't used Ai solutionarrow_forward5. A solution of sucrose is fermented in a vessel until the evolution of CO2 ceases. Then, the product solution is analyzed and found to contain, 45% ethanol; 5% acetic acid; and 15% glycerin by weight. If the original charge is 500 kg, evaluate; e. The ratio of sucrose to water in the original charge (wt/wt). f. Moles of CO2 evolved. g. Maximum possible amount of ethanol that could be formed. h. Conversion efficiency. i. Per cent excess of excess reactant. Reactions: Inversion reaction: C12H22O11 + H2O →2C6H12O6 Fermentation reaction: C6H12O6 →→2C2H5OH + 2CO2 Formation of acetic acid and glycerin: C6H12O6 + C2H5OH + H₂O→ CH3COOH + 2C3H8O3arrow_forwardShow work. don't give Ai generated solution. How many carbons and hydrogens are in the structure?arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

