
ORGANIC CHEMISTRY-ETEXT REG ACCESS
12th Edition
ISBN: 9781119308362
Author: Solomons
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16, Problem 18PP
Practice Problem 16.18
Triphenylphosphine can be used to convert
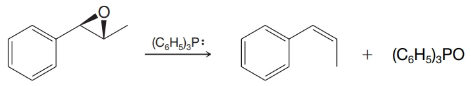
Propose a likely mechanism for this reaction.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Let's see if you caught the essentials of the animation.
What is the valence value of carbon?
a) 4
b) 2
c) 8
d) 6
A laser emits a line at 632.8 nm. If the cavity is 12 cm long, how many modes oscillate in the cavity? How long does it take for the radiation to travel the entire cavity? What is the frequency difference between 2 consecutive modes?(refractive index of the medium n = 1).
A laser emits a line at 632.8 nm. If the cavity is 12 cm long, how many modes oscillate in the cavity? How long does it take for the radiation to travel the entire cavity? What is the frequency difference between 2 consecutive modes?(refractive index of the medium n = 1).
Chapter 16 Solutions
ORGANIC CHEMISTRY-ETEXT REG ACCESS
Ch. 16 - PRACTICE PROBLEM 16.1 (a) Give IUPAC substitutive...Ch. 16 - Prob. 2PPCh. 16 - Prob. 3PPCh. 16 - Practice Problem 16.4
Provide the reagents and...Ch. 16 - Prob. 5PPCh. 16 - Prob. 6PPCh. 16 - Prob. 7PPCh. 16 - Prob. 8PPCh. 16 - Prob. 9PPCh. 16 - Practice Problem 16.10
Shown below is the...
Ch. 16 - Prob. 11PPCh. 16 - Practice Problem 16.12
What product would be...Ch. 16 - Prob. 13PPCh. 16 - Practice Problem 16.14
Dihydropyran reacts readily...Ch. 16 - Practice Problem 16.15 Show how you might use...Ch. 16 - Practice Problem 16.16 (a) Show how you might...Ch. 16 - Practice Problem 16.17
In addition to...Ch. 16 - Practice Problem 16.18
Triphenylphosphine can be...Ch. 16 - Prob. 19PPCh. 16 - PRACTICE PROBLEM 16.20
Give the structure of the...Ch. 16 - PRACTICE PROBLEM 16.21 What would be the major...Ch. 16 - Prob. 22PCh. 16 - 16.23 Write structural formulas for the products...Ch. 16 - Give structural formulas for the products formed...Ch. 16 - 16.25 What products would be obtained when...Ch. 16 - Predict the major organic product from each of the...Ch. 16 - 16.27 Predict the major product from each of the...Ch. 16 - 16.28 Predict the major product from each of the...Ch. 16 - Prob. 29PCh. 16 - 16.30 Write detailed mechanisms for each of the...Ch. 16 - Prob. 31PCh. 16 - Prob. 32PCh. 16 - Show how you would convert benzaldehyde into each...Ch. 16 - 16.34 Show how ethyl phenyl ketone could be...Ch. 16 - Show how benzaldehyde could be synthesized from...Ch. 16 - Give structures for compounds AE. Cyclohexanol...Ch. 16 - Prob. 37PCh. 16 - Prob. 38PCh. 16 - Prob. 39PCh. 16 - Prob. 40PCh. 16 - Prob. 41PCh. 16 - Prob. 42PCh. 16 - 16.43 The structure of the sex pheromone...Ch. 16 - Provide reagents that would accomplish each of the...Ch. 16 - Write a detailed mechanism for the following...Ch. 16 - Prob. 46PCh. 16 - Dutch elm disease is caused by a fungus...Ch. 16 - Prob. 48PCh. 16 - Compounds W and X are isomers; they have the...Ch. 16 - Compounds Y and Z are isomers with the molecular...Ch. 16 - Compound A (C9H18O) forms a phenylhydrazone, but...Ch. 16 - Compound B (C8H12O2) shows a strong carbonyl...Ch. 16 - Prob. 53PCh. 16 - Prob. 54PCh. 16 - Prob. 55PCh. 16 - (a) What would be the frequencies of the two...Ch. 16 - Prob. 57PCh. 16 - Prob. LGP
Additional Science Textbook Solutions
Find more solutions based on key concepts
Why is it necessary to be in a pressurized cabin when flying at 30,000 feet?
Anatomy & Physiology (6th Edition)
The number of named species is about __________, but the actual number of species on Earth is estimated to be a...
Biology: Life on Earth (11th Edition)
1. Which parts of the skeleton belong to the appendicular skeleton? Which belong to the axial skeleton?
Human Anatomy & Physiology (2nd Edition)
Thiols such as ethanethiol and propanethiol can be used to reduce vitamin K epoxide to vitamin KH2, but they re...
Organic Chemistry (8th Edition)
10.71 Identify each of the following as an acid or a base: (10.1)
H2SO4
RbOH
Ca(OH)2
HI
...
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Explain all answers clearly, with complete sentences and proper essay structure if needed. An asterisk (*) desi...
Cosmic Perspective Fundamentals
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The number of microstates corresponding to each macrostate is given by N. The dominant macrostate or configuration of a system is the macrostate with the greatest weight W. Are both statements correct?arrow_forwardFor the single step reaction: A + B → 2C + 25 kJ If the activation energy for this reaction is 35.8 kJ, sketch an energy vs. reaction coordinate diagram for this reaction. Be sure to label the following on your diagram: each of the axes, reactant compounds and product compounds, enthalpy of reaction, activation energy of the forward reaction with the correct value, activation energy of the backwards reaction with the correct value and the transition state. In the same sketch you drew, after the addition of a homogeneous catalyst, show how it would change the graph. Label any new line "catalyst" and label any new activation energy.arrow_forwardHow many grams of C are combined with 3.75 ✕ 1023 atoms of H in the compound C5H12?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Characteristic Reactions of Benzene and Phenols; Author: Linda Hanson;https://www.youtube.com/watch?v=tjEqEjDd87E;License: Standard YouTube License, CC-BY
An Overview of Aldehydes and Ketones: Crash Course Organic Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=-fBPX-4kFlw;License: Standard Youtube License