
Concept explainers
(a)
Interpretation:
The type of carbonyl group in erythrulose should be identified.
Concept introduction:
An aldehyde has an oxygen atom double bonded to carbon and a hydrogen atom bonded to the same carbon.
A ketone has oxygen atom double bonded to the C and there are no H atoms bonded to it.
In both aldehydes and ketones this carbon is known as carbonyl carbon.
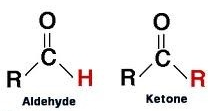
R − Other atoms or atomic groups connected to the compound apart from the carbonyl group.
(b)
Interpretation:
The 3 hydroxyl groups should be classified as primary (1
Concept introduction:
Depending on the number of hydrogen atoms attached to the carbon atom with hydroxyl group (-OH), they can be classified as primary (1
(c)
Interpretation:
Out of the 2 functional groups in erythrulose, which functional group is reacting with Tollens reagent should be considered.
Concept introduction:
Tollens reagent is amixture of a silver nitrate solution, ammonia and some sodium hydroxide. NaOH is added to maintain the pH in a basic level as the Tollensreaction only happens in a basic medium.
(d)
Interpretation:
Out of the 2 functional groups in erythrulose, which functional group is reacting with K2Cr2O7 should be considered.
Concept introduction:
K2Cr2O7 is an oxidizing agent. It has the ability to oxidize some functional groups of an organic compound.
Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
General, Organic, and Biological Chemistry - 4th edition
- Indicate the products of the reaction between CH3COCH2COONa (Sodium acetoacetate) and BrCH2COOC2H5arrow_forwardIndicate whether the product of the reaction between Naphthalene and CrO3 in acetic acid at 25ºC is 1,4 naphthoquinone or phthalic anhydride.arrow_forwardIndicate the products of the reaction between CH3COCH2COOC2H5 and Na+-OC2H5.arrow_forward
- Primary, Secondary, and Tertiary Alcohols O-H O-H O-H R₁-C-H R₁-C-H R₁-C-R₁ H R₂ R₂ Primary Alcohol Secondary Alcohol ChemistryLearner.com R stands for Carbon group like ethyl methyl propyl Tertiary Alcohol If 1 carbon group with two H attached to alcoholic carbon, then primary If 2 carbon group and 1 H are attached to alcoholic carbon, then secondary IF 3 carbon group and no H attach to alcoholic carbon then tertiary. The bottom line Starting "Weak" oxidant material PCC, DMP, Swern, etc Primary alcohol Aldehyde OH Secondary alcohol Ketone OH "Strong" oxidant KMnO4, H₂CrO4 (or equivalent) OH Carboxylic acid 요 Ketone No reaction No reaction Tertiary alcohol 1. Is ethanol a primary, secondary, or tertiary alcohol? Write out the structures of ethanol and any oxidation products of ethanol. If there is more than one oxidation product, give the structure of each of the products. 2. Is 2-propanol a primary, secondary, or tertiary alcohol? Write out the structures of 2-propanol and any…arrow_forwardFormulate the reaction: Naphthalene with CrO3 in acetic acid at 25ºCarrow_forwardComplete the reaction hand written pleasearrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


