
(a)
Interpretation:
The appropriate number with the quantity reactants needs to be matched.
Concept introduction:
An activated complex is formed as a result of collision between two particles with a sufficient amount of energy required for the collision. When the colliding particles break their bonds to form new bonds with the atoms of the particles it has undergo collision, a temporary transition state is generated which is an activated complex.
Answer to Problem 45A
Location 2 represents thereactants molecules.
Explanation of Solution
In the figure,
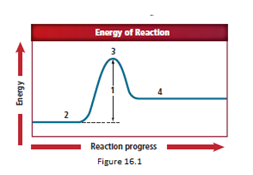
The energy diagram is shown for the endothermic reaction. In the beginning, the reactants begin at a lower energy than that of the products and the reactants absorbs adequate energy to overcome the activation energy barrier. After the crossing of the barrier, an activated complex is formed.
In a reaction, reactants are first thing that appear and it have the lowest energy in an exothermic reaction.
So, the correct answer for reactants is location 2.
(b)
Interpretation:
The appropriate number with the quantity activated complex needs to be matched.
Concept introduction:
An activated complex is formed as a result of collision between two particles with a sufficient amount of energy required for the collision. When the colliding particles break their bonds to form new bonds with the atoms of the particles it has undergo collision, a temporary transition state is generated which is an activated complex.
(b)
Answer to Problem 45A
Location 3 represents the activated complex.
Explanation of Solution
In the figure,
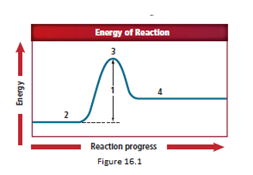
The energy diagram is shown for the endothermic reaction. In the beginning, the reactants begin at a lower energy than that of the products and the reactants absorbs adequate energy to overcome the activation energy barrier. After the crossing of the barrier, an activated complex is formed.
An activated complex is formed as a result of collision between two particles with a sufficient amount of energy required for the collision. After the crossing of the barrier, an activated complex starts forming.
So, as location 3 comes after location 2, the correct answer representing an activated complex is location 3.
(c)
Interpretation:
To match the appropriate number with the quantity products represents.
Concept introduction:
An activated complex is formed as a result of collision between two particles with a sufficient amount of energy required for the collision. When the colliding particles break their bonds to form new bonds with the atoms of the particles it has undergo collision, a temporary transition state is generated which is an activated complex.
(c)
Answer to Problem 45A
Location 4 represents the product.
Explanation of Solution
In the figure,
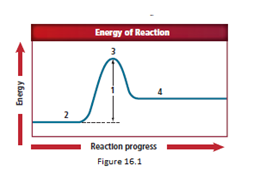
The energy diagram is shown for the endothermic reaction. In the beginning, the reactants begin at a lower energy than that of the products and the reactants absorbs adequate energy to overcome the activation energy barrier. After the crossing of the barrier, an activated complex is formed.
After the formation of the complex, the products start forming.
So, as location 4 comes after location 3, the correct answer representing an activated complex is location 4.
(d)
Interpretation:
To match the appropriate number with the quantity activation energyrepresents.
Concept introduction:
An activated complex is formed as a result of collision between two particles with a sufficient amount of energy required for the collision. When the colliding particles break their bonds to form new bonds with the atoms of the particles it has undergo collision, a temporary transition state is generated which is an activated complex.
(d)
Answer to Problem 45A
Location 1 represents the activation energy.
Explanation of Solution
In the figure,
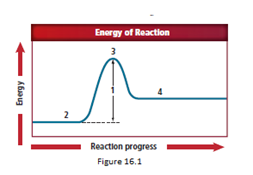
The energy diagram is shown for the endothermic reaction. In the beginning, the reactants begin at a lower energy than that of the products and the reactants absorbs adequate energy to overcome the activation energy barrier. After the crossing of the barrier, an activated complex is formed.
The activation energy value is the difference in energy value of reactants and activated complex.
Location 1 represents the activation energy, as the difference in energy is designated by location 1.
Chapter 16 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Campbell Biology in Focus (2nd Edition)
Biology: Life on Earth (11th Edition)
College Physics: A Strategic Approach (3rd Edition)
Campbell Essential Biology (7th Edition)
Microbiology: An Introduction
Human Anatomy & Physiology (2nd Edition)
- Part II. For the following compounds predict the no. Of signals expected for the 9) c) b d) C-NMR spectrum: لكمarrow_forwardIdentify the S and R configuration of all chiral centers.arrow_forward1) Draw the control charts for the following data and interpret the result and also develop control limts for future use. 24 samples are taken each with a subgroup size of 3. Don't Use the standard excel template and analyze.arrow_forward
- 1) Draw the control charts for the following data and interpret the result and also develop control limts for future use. 24 samples are taken each with a subgroup size of 3. Problem to be solved both as an assignment and laboratory. Subgroup X₁ X2 X3 1 7 8 10 2 9 9 14 3 15 16 10 4 14 13 15 5 12 11 10 6 10 11 9 I 7 10 9 9 8 15 17 13 9 10 7 8 10 9 8 9 11 8 8 10 12 17 13 10 13 10 12 11 14 9 9 10 15 10 8 8 16 11 10 9 17 10 10 8 18 8 9 7 19 9 8 9 22222 10 10 11 9 10 9 11 9 10 12 12 11 14 2012 4arrow_forwardHow much of each solution should be used to prepare 1L of a buffer solution with a pH of 9.45 using 3M Na2CO3 and 0.2M HCI? Given: Ka 1 = 4.3 × 10-7, Ka2 = 4.69 × 10-11arrow_forwardAdd substituents to draw the conformer below (sighting down the indicated bond), then rotate the back carbon to provide the anti staggered conformer. + H3C H Ph H Problem 25 of 30 Drawing Atoms, Bonds and Rings Charges Tap a node to see suggestions H H H Undo Rasat Remove Done Finish update Rotate Submitarrow_forward
- what temperature does a 50% (mole fraction) of ammonia/water liquid mixture boil at 1 atmarrow_forward1) Suppose 0.1 kg ice at 0°C (273K) is in 0.5kg water at 20°C (293K). What is the change in entropy of the ice as it melts at 0°? To produce the original "water gas" mixture, carbon (in a combustible form known as coke) is reacted with steam: 131.4 kJ + H20(g) + C(s) → CO(g) + H2(g) From this information and the equations in the previous problem, calculate the enthalpy for the combustion or carbon to form carbon dioxide. kindly show me how to solve both parts of the same long problem. Thanksarrow_forwardwe were assigned to dilute 900ppm in to 18ppm by using only 250ml vol flask. firstly we did calc and convert 900ppm to 0.9 ppm to dilute in 1 liter. to begin the experiment we took 0,225g of kmno4 and dissolved in to 250 vol flask. then further we took 10 ml sample sol and dissolved in to 100 ml vol flask and put it in to a spectrometer and got value of 0.145A . upon further calc we got v2 as 50ml . need to find DF, % error (expval and accptVal), molarity, molality. please write the whole report. thank you The format, tables, introduction, procedure and observation, result, calculations, discussion and conclusionarrow_forward
- Q5. Predict the organic product(s) for the following transformations. If no reaction will take place (or the reaction is not synthetically useful), write "N.R.". Determine what type of transition state is present for each reaction (think Hammond Postulate). I Br₂ CH3 F2, light CH3 Heat CH3 F₂ Heat Br2, light 12, light CH3 Cl2, light Noarrow_forwardNonearrow_forwardIn the phase diagram of steel (two components Fe and C), region A is the gamma austenite solid and region B contains the gamma solid and liquid. Indicate the degrees of freedom that the fields A and B have,arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





