
Use the diagram below to answer Questions 8 and 9.
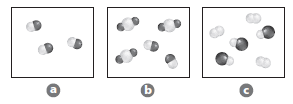
Which sample could contain particles of magnesiumfluoride?
A. a
B. b
C. c
D. Both a and b
Interpretation:
The sample that contain particles a of magnesium fluoride from the given figure needs to be determined.
 Concept introduction:
Concept introduction:
Diatomic molecules consists two atoms of different or same elements. For example,
Answer to Problem 9STP
B.b
Explanation of Solution
B. magnesium fluoride has three atoms - one magnesium and two fluorine atoms. Two fluorine atoms are attached to the magnesium atom. The given figure shows different size and colors. Option B has three atoms which attached to each other with larger central atom and two atoms are the same in color.
The incorrect options are as follows:
A. a
Figure a has different atoms that attached one another.
C. c
Figure c has different atoms and two identical atoms that attached one another.
D. both a and b
Both figure a has different atoms.
Chapter 16 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Concepts of Genetics (12th Edition)
Campbell Biology: Concepts & Connections (9th Edition)
Chemistry: A Molecular Approach (4th Edition)
Biology: Life on Earth (11th Edition)
Organic Chemistry (8th Edition)
Campbell Biology (11th Edition)
- how many moles of H2O2 are required to react with 11g of N2H4 according to the following reaction? (atomic weights: N=14.01, H=1.008, O= 16.00) 7H2O2 + N2H4 -> 2HNO3 + 8H20arrow_forwardcalculate the number of moles of H2 produced from 0.78 moles of Ga and 1.92 moles HCL? 2Ga+6HCL->2GaCl3+3H2arrow_forwardan adult human breathes 0.50L of air at 1 atm with each breath. If a 50L air tank at 200 atm is available, how man y breaths will the tank providearrow_forward
- Using reaction free energy to predict equilibrium composition Consider the following equilibrium: 2NO2 (g) = N2O4(g) AGº = -5.4 kJ Now suppose a reaction vessel is filled with 4.53 atm of dinitrogen tetroxide (N2O4) at 279. °C. Answer the following questions about this system: Under these conditions, will the pressure of N2O4 tend to rise or fall? Is it possible to reverse this tendency by adding NO2? In other words, if you said the pressure of N2O4 will tend to rise, can that be changed to a tendency to fall by adding NO2? Similarly, if you said the pressure of N2O4 will tend to fall, can that be changed to a tendency to '2' rise by adding NO2? If you said the tendency can be reversed in the second question, calculate the minimum pressure of NO 2 needed to reverse it. Round your answer to 2 significant digits. 00 rise ☐ x10 fall yes no ☐ atm G Ar 1arrow_forwardWhy do we analyse salt?arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. H H CH3OH, H+ H Select to Add Arrows H° 0:0 'H + Q HH ■ Select to Add Arrows CH3OH, H* H. H CH3OH, H+ HH ■ Select to Add Arrows i Please select a drawing or reagent from the question areaarrow_forward
- What are examples of analytical methods that can be used to analyse salt in tomato sauce?arrow_forwardA common alkene starting material is shown below. Predict the major product for each reaction. Use a dash or wedge bond to indicate the relative stereochemistry of substituents on asymmetric centers, where applicable. Ignore any inorganic byproducts H Šali OH H OH Select to Edit Select to Draw 1. BH3-THF 1. Hg(OAc)2, H2O =U= 2. H2O2, NaOH 2. NaBH4, NaOH + Please select a drawing or reagent from the question areaarrow_forwardWhat is the MOHR titration & AOAC method? What is it and how does it work? How can it be used to quantify salt in a sample?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





