
(a)
Interpretation:
It should be determined that the structure of the ammonium ion formed, when the given amine is treated with an acid.
Concept introduction:
In chemistry Structure is the arrangement of
Depending on the number of carbon side chain of the nitrogen, different types of amines can form.
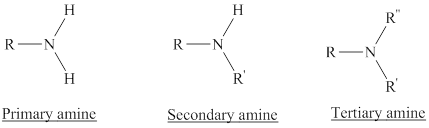
From the name of the compound its structure can be determined.
Primary amines can be named in the IUPAC system in several ways,
For simple amines the suffix – amine is added to the name of the alkyl substituent.
The suffix-amine can be used in place of the final –e in the name of the parent compound.
For a secondary amine an N prefixes the compound giving the shorter carbon chain and its chain prefix name.
For a tertiary amine an N, N prefixes the compound giving the two shorter carbon chains and their side chain prefix names.
Reaction of amines and acid will give amine salt an (ammonium ion).
(b)
Interpretation:
It should be determined that the structure of the ammonium ion formed, when the given amine is treated with an acid.
Concept introduction:
In chemistry Structure is the arrangement of chemical bonds between atoms in a molecule, specifically which atoms are chemically bonded to what other atoms with what kind of chemical bond.
Amines are the derivatives of ammonia
Depending on the number of carbon side chain of the nitrogen, different types of amines can form.

From the name of the compound its structure can be determined.
Primary amines can be named in the IUPAC system in several ways,
For simple amines the suffix – amine is added to the name of the alkyl substituent.
The suffix-amine can be used in place of the final –e in the name of the parent compound.
For a secondary amine an N prefixes the compound giving the shorter carbon chain and its chain prefix name.
For a tertiary amine an N, N prefixes the compound giving the two shorter carbon chains and their side chain prefix names.
Reaction of amines and acid will give amine salt an (ammonium ion).
(c)
Interpretation:
It should be determined that the structure of the ammonium ion formed, when the given amine is treated with an acid.
Concept introduction:
In chemistry Structure is the arrangement of chemical bonds between atoms in a molecule, specifically which atoms are chemically bonded to what other atoms with what kind of chemical bond.
Amines are the derivatives of ammonia
Depending on the number of carbon side chain of the nitrogen, different types of amines can form.

From the name of the compound its structure can be determined.
Primary amines can be named in the IUPAC system in several ways,
For simple amines the suffix – amine is added to the name of the alkyl substituent.
The suffix-amine can be used in place of the final –e in the name of the parent compound.
For a secondary amine an N prefixes the compound giving the shorter carbon chain and its chain prefix name.
For a tertiary amine an N, N prefixes the compound giving the two shorter carbon chains and their side chain prefix names.
Aniline is an
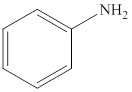
Reaction of amines and acid will give amine salt an (ammonium ion).
Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
FUND.OF GEN CHEM CHAP 1-13 W/ACCESS
- In a diffraction experiment of a native crystal, intensity of reflection (-1 0 6) is equivalent to the intensity of reflection (1 0 -6). true or false?arrow_forwardin an x-ray diffraction experiment, moving the detector farther away from the crystal will allow collection of reflection of reflections with high Miller indices. true or false?arrow_forwardShow the mechanism for the acid-catalyzed formation of an [α-1,6] glycosidic linkagebetween two molecules of α-D-glucopyranose.arrow_forward
- Label the following polysaccharide derivatives as reducing or nonreducing. a. C. b. HO CH₂OH CH2OH OH OH OH OH OH HOCH₂ OH OH OH HOCH₂ HO HO HO OH OH ΙΟ CH₂OH OH OH "OH OHarrow_forwardFor a red blood cell (erythrocyte) undergoing active glycolysis, briefly explain how increases in concentration of the following factors are likely to affect glycolytic flux. a. ATP b. AMP c. F-1,6-BP d. F-2,6-BP e. Citrate f. Glucose-6-phosphatearrow_forwardThe ∆G°’ for hydrolysis of phosphoenol pyruvate is -62.2 kJ/mol. The standard freeenergy of ATP hydrolysis is -30.5 kJ/mol. A. What is the standard free energy and K eq of the spontaneous reaction betweenADP/ATP and phosphoenol pyruvate. B. Repeat A for F-1,6-BP (∆G°’=-16.7 kJ/mol) and 1,3-BPG (∆G°’=-49.6 kJ/mol)hydrolysis. C. If the ATP and ADP concentrations are 8mM and 1mM respectively, what would bethe ratio of pyruvate/phosphoenolpyruvate at equilibrium?arrow_forward
- Answerarrow_forward13. Which one is the major organic product of the following sequence of reactions? A OH (CH3)2CHCH2COOH SOCI2 CH3OH 1. CH3MgBr 2. H₂O, H+ B C D OH E OHarrow_forward14. Which one is the major organic product of the following sequence of reactions? (CH3)2CH-COCI CH3OH 1. DIBALH, -78°C 1. PhCH2MgBr ? 2. H2O, HCI 2. H2O, HCI OH OMe A Ph B Ph OH Ph C OMe Ph D E OH .Pharrow_forward
- 6. Which one is the major organic product obtained from the following reaction? CO₂Me 1. LiAlH4 2. H₂O CH₂OH CH₂OCH3 5555 HO A B HO C HO D CH₂OH E ?arrow_forward1. (10 points) Pulverized coal pellets, which may be ° approximated as carbon spheres of radius r = 1 mm, are burned in a pure oxygen atmosphere at 1450 K and 1 atm. Oxygen is transferred to the particle surface by diffusion, where it is consumed in the reaction C + O₂ →> CO₂. The reaction rate is first order and of the form No2 = k₁C₁₂(r), where k₁ = 0.1 m/s. Neglecting changes in r, determine the steady-state O₂ molar consumption rate in kmol/s. At 1450 K, the binary diffusion coefficient for O2 and CO2 is 1.71 x 10ª m²/s.arrow_forward2. (20 points) Consider combustion of hydrogen gas in a mixture of hydrogen and oxygen adjacent to the metal wall of a combustion chamber. Combustion occurs at constant temperature and pressure according to the chemical reaction 2H₂+ O₂→ 2H₂O. Measurements under steady-state conditions at 10 mm from the wall indicate that the molar concentrations of hydrogen, oxygen, and water vapor are 0.10, 0.10, and 0.20 kmol/m³, respectively. The generation rate of water vapor is 0.96x102 kmol/m³s throughout the region of interest. The binary diffusion coefficient for each of the species (H, O̟, and H₂O) in the remaining species is 0.6 X 10-5 m²/s. (a) Determine an expression for and make a qualitative plot of C as a function of distance from the wall. H2 (b) Determine the value of C2 at the wall. H2 (c) On the same coordinates used in part (a), sketch curves for the concentrations of oxygen and water vapor. This will require you to calculate Co, and C. 02 H20 (d) What is the molar flux of water…arrow_forward
 Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College
Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning
Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning





